��Ŀ����
����Ŀ��ijС����CoCl2��6H2O��NH4Cl��H2O2��Һ�����Ȼ��Ϊԭ�ϣ��ڻ���̿���ºϳ��˳Ȼ�ɫ����X��Ϊ�ⶨ����ɣ���������ʵ�顣
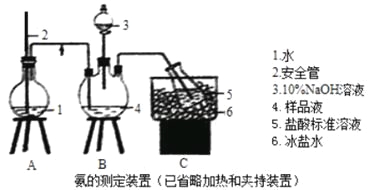
�����IJⶨ����ȷ��ȡwgX��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������Ʒ��Һ�еİ�ȫ����������V1mLc1mol��L��1��������Һ���ա�����������ȡ�½���ƿ����c2mol��L��1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2mLNaOH��Һ��
���ȵIJⶨ��ȷ��ȡ��ƷX�������Һ����AgNO3����Һ�ζ���K2CrO4��ҺΪָʾ����������ש��ɫ����������ʧΪ�յ㣨Ag2CrO4Ϊש��ɫ����
�ش��������⣺
��1��װ���а�ȫ�ܵ�����ԭ����_________��
��2����NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ��_____ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ________��
��3����Ʒ�а���������������ʽΪ____________��
��4���ⶨ��ǰӦ�ö�װ�ý��������Լ��飬�������Բ��òⶨ�����___������ƫ��������ƫ��������
��5���ⶨ�ȵĹ����У�ʹ����ɫ�ζ��ܵ�ԭ����___________���ζ��յ�ʱ������Һ��c(Ag��)=2.0��10��5mol��L��1��c(CrO42��)Ϊ______mol��L��1������֪��Ksp(Ag2CrO4)=1.12��10��12��
��6�����ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1:6:3���ܵĻ��ϼ�Ϊ______���Ʊ�X�Ļ�ѧ����ʽΪ____________________��X���Ʊ��������¶Ȳ��ܹ��ߵ�ԭ����__________________��
���𰸡���A��ѹ������ʱ����ȫ����Һ��������ʹAƿ��ѹ���ȶ� �� ��̪������죩 (c1V1��c2V2)��10��3��17/w��100% ƫ�� ��ֹ����������ֽ� 2.8��10��3 +3 2CoCl2+2NH4Cl+10NH3+H2O2===2[Co(NH3)6]Cl3+2H2O �¶ȹ��߹�������ֽ⡢�����ݳ�
��������
��1����������ƿ��ѹǿ������С�����������Σ�գ�������A�ڵ�����Һ�����ߣ�������ѹ��������С��������ͨ�����ܽ�����ƿ��Ҳ������ɵ�������ȫ���õ�ԭ����ʹA��ѹǿ�ȶ���
��2����ֻ��ʢ���ڼ�ʽ�ζ����У�������Һֻ��ʢ������ʽ�ζ����У�������NaOH����Һȷ����ʣ��HClʱ��Ӧʹ�ü�ʽ�ζ���ʢ��NaOH��Һ��NaOH��Һ��������Һǡ�÷�Ӧ������ԣ�����ѡ�����Ի���Ա�ɫ��Χ�ڵ�ָʾ��������Ϊ���Ա�ɫָʾ������̪Ϊ���Ա�ɫָʾ�������Կ���ѡȡ���Ȼ��̪��ָʾ����
��3���백����Ӧ��n��HCl����V1��10-3L��c1molL-1-c2molL-1��V2��10-3L����c1V1-c2V2����10-3mol�����ݰ�����HCl�Ĺ�ϵʽ֪��n��NH3����n��HCl������c1V1-c2V2����10-3mol����������������![]() ��
��
��4���������Բ��ã����²��ְ���й©����������������ƫ�͡�
��5�����������ȶ��������ֽ⣬Ϊ��ֹ�������ֽ⣬����ɫ�ζ���ʢ����������Һ�����ݸ��������ܶȻ�������֪c��CrO42-��=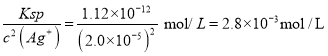 ��
��
��6�����ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3�����仯ѧʽΪ[Co��NH3��6]Cl3�����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��CoԪ�ػ��ϼ�Ϊ+3�ۣ��÷�Ӧ��Coʧ���ӡ�˫��ˮ�õ��ӣ�CoCl26H2O��NH4Cl��H2O2��NH3������Ӧ����[Co��NH3��6]Cl3��ˮ����Ӧ����ʽΪ2CoCl2��2NH4Cl��10NH3��H2O2=2[Co(NH3)6]Cl3��2H2O��˫��ˮ�ֽ⡢������ܽ�������¶ȵ����߶����ͣ�����X���Ʊ��������¶Ȳ��ܹ��ߡ�
�����硿
��ȷ������ʵ����ʺ�ʵ��ԭ���ǽ��Ĺؼ���ע���ۺ�ʵ�������Ľ���˼·������1�������⣬��ȷʵ���Ŀ�ĺ�ԭ����ʵ��ԭ���ǽ��ʵ����ĺ��ģ���ʵ����Ƶ����ݺ���㡣ʵ��ԭ���ɴ�����Ļ�ѧ�龰(����������ʵ��Ŀ��)�����Ԫ�ػ�������й�֪ʶ��ȡ���ڴ˻����ϣ���ѭ�ɿ��ԡ�����ԡ���ȫ�Ե�ԭ��ȷ������ʵ��Ŀ�ġ�Ҫ��ķ�������2������̣�����ʵ��������Ⱥ�˳����ʵ��ԭ����ȷ����ʵ�鷽���е�ʵ����̣�ȷ��ʵ������ķ������裬���ո���ʵ�������Ҫ�㣬����ʵ��������Ⱥ�˳��3����ͼ����������ʵ��װ�õ����á��������ۺ�ʵ����ͼ�Ľ�ϣ�˼���������ڷ����������У�Ҫ����ϸ�µط���ͼ����ʾ�ĸ���װ�ã������ʵ��Ŀ�ĺ�ԭ����ȷ�������ڸ�ʵ���е����á���4��ϸ�������ó���ȷ��ʵ����ۡ�ʵ������(������)�ǻ�ѧԭ�������ڱ��֡��ڷ���ʵ������(������)�Ĺ����У�Ҫ�����ҳ�Ӱ��ʵ��ɰܵĹؼ��Լ���������ԭ����й������й��ɳ�������ʽ�����Ʊ仯���ߵȡ�

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�����Ŀ����ʽ������(NiOOH)�����������ص��������ϣ����÷�����������Ҫ��Ni��Al������Cr��FeS ��)���Ʊ����乤���������£�

�ش��������⣺
(1)�����ݳ�����ʱ��������Ӧ�����ӷ�Ӧ����ʽΪ_________________________;
(2)���ܽ⡱ʱ�ų�������Ϊ_______________ (�ѧʽ);
(3)��֪�������½������ӿ�ʼ��������ȫ������pH���±���
��ʼ������pH | ��ȫ������pH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
����pH 1��ʱ����ҺpH��ΧΪ______________________��
(4)�ڿ����м���Ni(OH)2�ɵ�NiOOH,��д���˷�Ӧ�Ļ�ѧ����ʽ_____________;

(5)����������Һ���ж��ִ�����ʽ, CrO42����Cr2O72������Һ�п��ת���������£���ʼŨ��Ϊ1.0mol/L��Na2CrO4��Һ��c(Cr2O72��)��c(H+)�ı仯��ͼ��ʾ�������ӷ���ʽ��ʾNa2CrO4��Һ�е�ת����Ӧ________________������A�����ݼ������ת����Ӧ��ƽ�ⳣ��Ϊ______________,�¶����ߣ���Һ��CrO42����ƽ��ת���ʼ�С����÷�Ӧ�ġ�H____0���>������<����=������
����Ŀ����������������ˮ����ζ������������ˮ�㾫�����쾫�ͣ�Ҳ��������ʳƷ�У�ʵ�����Ʊ��������£�
�������������ֲ�Ʒ���Ʊ�
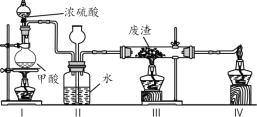
�ڸ����50mL����Բ����ƿ�У�����6.1g�����ᡢ������ˮ�Ҵ���8mL����1mLŨ���ᣬҡ�Ⱥ�ӷ�ʯ����װ��ˮ����ˮԡ���ȣ���ʼ���ƻ����ٶ�1��2d/s����Ӧʱ�����Ҵ���ˮ���γ�����������������������Ȼ���Լ1Сʱ������ˮ���в�����Сˮ������ʱ��ֹͣ���ȡ���Ϊ����װ�ã����������Ҵ��ͱ���
�е㣨���� | ��Է������� | |
������ | 249 | 122 |
���������� | 212.6 | 150 |
��1��д������������18O��ǵ��Ҵ�������Ӧ�ķ���ʽ___________________��
��2������A�����Ƽ���ˮͨ���_________________________��
��3��ͨ����ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��Ŀ����___________________��
��4��ʵ�������û�мӷ�ʯ����д�����ӷ�ʯ�IJ���___________________��
��5��ͼ2װ���еĴ���______________________��
���������������ľ���
�������������ֲ�Ʒ�����·������о��ƣ�����ƿ�е�Һ�嵹��ʢ��30mL��ˮ���ձ��У������·������뱥��Na2CO3��Һ������ϸ�ķ�ĩ�����������������pH��ֽ����²���Һ�����ԡ��÷�Һ©���ֳ��ֲ��ˮ�������ѣ�10mL����ȡ���ϲ��л��࣬����ˮCaCl2��������Ĵֲ�������ˮԡ�������ѣ��ٸ��ü�ѹ�����ռ���֡�
��6�����뱥��̼������Һ���˿��Խ��ͱ����������ܽ���⣬���е�������__________��
��7�����վ�����IJ������У���ò�Ʒ���Ϊ5mL�������������ܶ�Ϊ1.05g��cm3����ʵ��IJ���Ϊ_________��
����Ŀ��SO2�Ĵ������ǹ�ҵ��ȡ����Ĺؼ�����֮һ��2SO2 + O2![]() 2SO3 ���ݻ���Ϊ2L������ͬ������a��b��c��d��e����ܱ������о�����2molSO2��1molO2������壬���Ʋ�ͬ�ķ�Ӧ�¶ȡ���Ӧ����5minʱ���������������±���
2SO3 ���ݻ���Ϊ2L������ͬ������a��b��c��d��e����ܱ������о�����2molSO2��1molO2������壬���Ʋ�ͬ�ķ�Ӧ�¶ȡ���Ӧ����5minʱ���������������±���
�����¶� ���ʵ���(mol) | a���� 400�� | b���� 425�� | c���� 450�� | d���� 475�� | e���� 500�� |
O2 | x | 0.6 | 0.3 | 0.5 | 0.7 |
SO3 | y | 0.8 | 1.4 | 1.0 | 0.6 |
(1)5minʱ���a�����л����������ʵ���������0.2mol������5min��SO3��ƽ����Ӧ����____________��
(2)��Ӧ���е�5minʱ��b�����еķ�Ӧ�Ƿ�ﵽƽ��״̬��_______(����������������)�������ǣ�_____________________________________________________________________________��
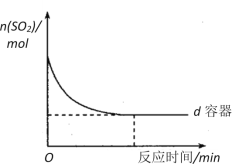
(3)��ͼ�л���e������SO2���ʵ�����ʱ��ı仯����_______________________��
(4)��ҵ�ϳ���Na2SO3��Һ������SO2���÷�Ӧ�����ӷ���ʽ�ǣ�___________________��
(5)��֪NaHSO3��Һ�����ԣ���Һ��c(H2SO3)_______ c(SO32��)(ѡ�������=)��NaHSO3���ܺ�ǿ�ᷴӦҲ�ܺ�ǿ����Һ��Ӧ����ƽ���ƶ�ԭ��˵��NaHSO3�ܺ�ǿ����Һ��Ӧ��ԭ��_______________