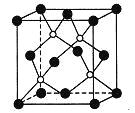
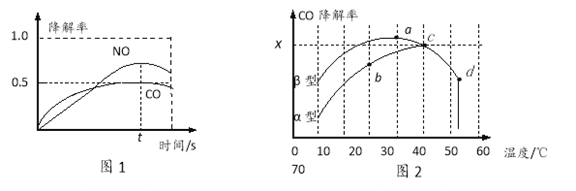
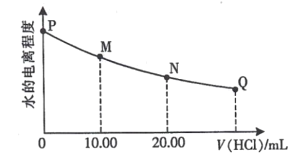
��Ŀ����
����Ŀ����MgCl2��AlCl3�Ļ����Һ�У���ʼ�μ��Լ�X��֮��ĵ��Լ�Y�����ó��������ʵ���n(mol)���Լ����V(mL)��Ĺ�ϵ��ͼ��ʾ�����½��۴������(����)

A.X�����ᣬY��NaOH��Һ����c(Y)��2c(X)
B.ԭ���Һ�У�c(Al3��)��c(Mg2��)��c(Cl��)��1��1��5
C.X��NaOH��Һ��Y�����ᣬ��c(NaOH)��c(HCl)��2��1
D.��b��c��Ӧ�����ӷ���ʽΪH����OH��===H2O
���𰸡�A
��������
����ͼ���֪�ȼ�����Լ�Xһ��ʼ�Ͳ��������������Լ��ļ�����������ܽ⣬�����Լ�XΪNaOH������������Լ�Y������������������������ļӦ���������Լ�Y��������������ƫ���������ᷴӦ���ɳ���������������ƫ������ȫ��ת��Ϊ�����������ٵ����ᣬ���ܽ�������������������þ�������Լ�YΪ���ᡣ
A. �������Ϸ�����֪X���������ƣ�Y�����ᣬ��A����
B. ���������Ƶ�Ũ��Ϊ6mol/l���Ӽ���5mLX���ɳ�����࣬�ټ�����1mLX���������ٵ���Сֵ���μ�����������5��6��1 mL��ʱ��Ӧ��Ӧ�����ӷ���ʽΪ��Al��OH��3��+NaOH=NaAlO2+2H2O���ɴ˿�֪��n��Al3+��=n[Al��OH��3]=0.006mol����ǰ5mLNaOH�γ�����������֪��2n��Mg2+��+3n��Al3+��=0.03mol������n��Mg2+��=0.006mol����Һ�������������������ȣ���n��Cl-��=0.03mol������Һ��c(Al3��)��c(Mg2��)��c(Cl��)��1��1��5����B��ȷ��
C. ��ͼ��֪��5��6��1mL��Ϊ�����������ܽ⣬������Ӧ��Al��OH��3+NaOH=NaAlO2+2H2O��9��11��2 mL��Ϊƫ������ǡ����ȫ����Al��OH��3������������Ӧ��NaAlO2+HCl+H2O=Al��OH��3��+NaCl��������Ԫ���غ�֪��NaOH��Al��OH��3��AlO2-��H+�ɴ˿����Ƴ���n��NaOH��=n��HCl������Ϊv��NaOH����v��HCl��=1��2�����ԣ�c��NaOH����c��HCl��=2��1����C��ȷ��
D. ��6mL����ԭ���ĵμ�NaOH����Ϊ�μ����ᣬ��ͼ��֪7mL��9mLʱ��ӦҺ���г��������䣬������֪��ʱ������������кͷ�Ӧ����H����OH��===H2O����D��ȷ��
��ĿҪ��ѡ����ģ���ѡA��