��Ŀ����
6�� �������ƴ�������Ⱦ�Ϻ��л��ϳɹ�ҵ����ľм�Ʊ��������Ƶ��������£�
�������ƴ�������Ⱦ�Ϻ��л��ϳɹ�ҵ����ľм�Ʊ��������Ƶ��������£�
��֪���������У����Ʒ�ӦҺ���¶���55��60�������·�������Ҫ��ӦΪ��
C6H12O6+12HNO3$\frac{\underline{\;��\;}}{\;}$3H2C2O4+9NO2��+3NO��+9H2O
��1�����ܹ����У�����������Ǵ���ά�ص�ˮ��õ������ǣ�������ɺ�����Һ����Ũ������ȴ�ᾧ�����ˣ���������ƣ���ϴ�ӵõ���ɫ��״���������ƣ�
��2��ʵ����ģ�����������չ��̵�װ����ͼ��ʾ��Aװ������Ʒ�ӦҺ���¶Ȳ�����60���ԭ�����¶ȹ��ᵼ������ķֽ⣬ʹNaNO2���ʽ��ͣ�Bװ�������Ʊ��������ƣ�����ʢ�ŵ���Һ��b������ĸ����
a��NaCl��Һ b��Na2CO3��Һ c��NaNO3��Һ
��3����ҵ�������չ����������NO��NO2�����ʵ����Ƚӽ�1��1������NO����n��NO2����1��1����ᵼ���ŷ�������NO�������ߣ���n��NO����n��NO2����1��1��ʹ��Ʒ�л��е�����ΪNaNO3��
��4����֪NaNO2�ܰ����������µ�Fe2+������ͬʱ����һ���ж������壬��д���÷�Ӧ�����ӷ���ʽ��NO2-+2H++Fe2+=NO��+Fe3++H2O��
��5����֪����NaNO2�������ԣ��������������ܰ�I-����ΪI2��S2${{O}_{3}}^{2-}$���ܰ�I2��ԭΪI-����NaNO2Ҳ�л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��Ϊ�ⶨ��ƷNaNO2�Ĵ��ȣ��벹������ʵ�鷽����ȷ����������NaNO2��Ʒ������ƿ�У�������ˮ�ܽ⣬Ȼ����c3mol•L-1����KMnO4��Һ�ζ�����Һ����ɫǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ���������ظ����ϲ���2��3�Σ�ʵ���пɹ�ѡ����Լ���ϡ���ᡢc1 mol•L-1 KI��Һ��������Һ��c2 mol•L-1 Na2S2O3��Һ��c3 mol•L-1����KMnO4��Һ��
���� ��1��ľм����Ҫ�ɷ�����ά��[��C6H10O5��n]�������������Ӧ�������Ƿ�Ӧ�����Լ�H2SO4��Ŀ�Ĵ���ά�ص�ˮ��õ���������Һ��
����Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
��2��HNO3���ȶ����¶ȹ��߷����ֽ⣻��NaOH���յ�ԭ���ǣ�2NaOH+NO+NO2=2NaNO2�����ڼ�����Һ��NO2��NO�������з�Ӧ��
��3����NO���࣬��NO���ܱ����գ��������������Ⱦ����NO2�������Ӧ��2NO2+2NaOH=NaNO3+NaNO2+H2O��
��4��NaNO2��Fe2+��������������ԭ����Ԫ�ػ��ϼ۽��ͣ������ж����壬˵������ԭΪNO���������������ݵ�ʧ�����غ���ƽ��Ӧ��
��5������KMnO4����NO2-���еζ�����NaNO2�ĺ��������ɲ�������������NO2-����I-����I2������Na2S2O3�ζ����㣬��Ϊ��Ʒ�е�NaNO3�е�NO3-����������Ҳ������I-����I2�����ظ��ζ��Լ�����
��� �⣺��1��ľм����Ҫ�ɷ�����ά��[��C6H10O5��n]�������������Ӧ�������Ƿ�Ӧ�����Լ�H2SO4��Ŀ�ģ�����ά�ص�ˮ��õ���������Һ��
����Һ�л��NaNO2���壬��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
�ʴ�Ϊ������ά�ص�ˮ��õ������ǣ���ȴ�ᾧ�����ˣ�
��2���¶ȹ��ߣ�HNO3�ᷢ���ֽ⣬ʹNaNO2���ʽ��ͣ���NaOH���յ�ԭ���ǣ�2NaOH+NO+NO2=2NaNO2�����ڼ�����Һ��NO2��NO�������з�Ӧ������ѡ��Na2CO3��Һ��
�ʴ�Ϊ���¶ȹ��ᵼ������ķֽ⣬ʹNaNO2���ʽ��ͣ�b��
��3����NO���࣬��NO���ܱ����գ��������������Ⱦ����NO2�������Ӧ��2NO2+2NaOH=NaNO3+NaNO2+H2O������NaNO3���ʣ�
�ʴ�Ϊ���ŷ�������NO�������ߣ�NaNO3��
��4��NaNO2��Fe2+��������������ԭ����Ԫ�ػ��ϼ۽��ͣ������ж����壬˵������ԭΪNO���÷�Ӧ�����ӷ���ʽ��NO2-+2H++Fe2+=NO��+Fe3++H2O��
�ʴ�Ϊ��NO2-+2H++Fe2+=NO��+Fe3++H2O��
��5������KMnO4����NO2-���еζ�����NaNO2�ĺ��������ɲ�������������NO2-����I-����I2������Na2S2O3�ζ����㣬��Ϊ��Ʒ�е�NaNO3�е�NO3-����������Ҳ������I-����I2������ʵ�鷽����ȷ����������NaNO2��Ʒ������ƿ�У�������ˮ�ܽ⣬Ȼ����c3mol•L-1����KMnO4��Һ�ζ�����Һ����ɫǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ���������ظ����ϲ���2��3�Σ�
�ʴ�Ϊ��Ȼ����c3mol•L-1����KMnO4��Һ�ζ�����Һ����ɫǡ�ñ�Ϊdz��ɫ���Ұ�����ڲ���ɫ���������ظ����ϲ���2��3�Σ�
���� ���⿼���������Ƶ��Ʊ�ʵ�飬���ؿ���ѧ����ԭ�������⣬�Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

| A�� | ���ڽ���Ԫ�� | |
| B�� | ��������������֮��Ϊ50 | |
| C�� | ԭ�ӵĺ����������39 | |
| D�� | ${\;}_{39}^{49}$Y��${\;}_{39}^{50}$Y�����ֲ�ͬ�ĺ��� |
ʵ��ԭ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+10CO2��+8H2O
ʵ�����ݼ���¼
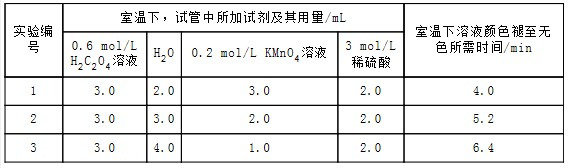
��ش�
��1�������ϱ��е�ʵ�����ݣ����Եõ��Ľ���������������ͬʱ������KMnO4Ũ�ȷ�Ӧ��������
��2������ʵ��1�����ݼ��㣬����KMnO4��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ���ԣ�KMnO4��=1.5��10-2mol/��L•min����
��3����С��ͬѧ���ݾ��������n��Mn2+����ʱ��仯���Ƶ�ʾ��ͼ����ͼ1��ʾ������ͬѧ�������е�ʵ�����Ϸ��֣���ʵ�������n��Mn2+����ʱ��仯������Ӧ��ͼ2��ʾ����С��ͬѧ����ͼ2��ʾ��Ϣ������µļ��裬����������ʵ��̽����
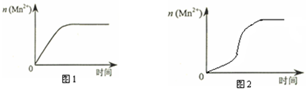
�ٸ�С��ͬѧ����ļ������������е�MnSO4Ϊ�÷�Ӧ�Ĵ�������Mn2+�Ը÷�Ӧ�д����ã���
�����������С��ͬѧ���ʵ�鷽��������д���пհף�
| ʵ���� | �����£��Թ��������Լ���������/mL | �����Թ��м����������� | ��������Һ��ɫ������ɫ����ʱ��/min | |||
| 0.6 mol/L H2C2O4��Һ | H2O | 3 mol/L ϡ���� | 0.05 mol/L KMnO4��Һ | |||
| 4 | 3.0 | 2.0 | 2.0 | 3.0 | t | |
��4����ҵ�Ͽ��õ��K2MnO4Ũ��Һ�ķ�����ȡKMnO4������ʱ�����������ĵ缫��ӦΪMnO42--e-=MnO4-���ܷ���ʽΪ2K2MnO4+2H2O�T2KMnO4+2KOH+H2����
| A�� | Al3+ | B�� | [Al��OH��4]- | C�� | HCO3- | D�� | SiO32- |
| A�� | H2��D2��Ϊͬλ�� | B�� | 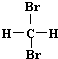 �� ��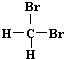 ��Ϊͬ���칹�� ��Ϊͬ���칹�� | ||
| C�� | H��D��T����Ϊͬ�������� | D�� | 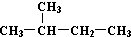 �� �� 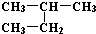 ��ͬһ������ ��ͬһ������ |

| A�� | ���������Ǻ��зǼ��Լ������ӻ����� | |
| B�� | ����Ļ�ѧʽΪBa2O2 | |
| C�� | �þ��徧���ṹ��CsCl���� | |
| D�� | ������Ba2+����λ��Ϊ8 |
| A�� | ������Ӧ�У�N2�ǻ�ԭ����Al2O3�������� | |
| B�� | AlN��Ħ������Ϊ41 g | |
| C�� | AlN�е�Ԫ�صĻ��ϼ�Ϊ+3 | |
| D�� | ������Ӧ�У�ÿ����1 mol AlN��ת��3 mol���� |
