��Ŀ����
20�������й���Һ����Ũ�ȹ�ϵ������������ǣ�������| A�� | 0.1 mol•L-1 KHC2O4��Һ�У�c��OH-��=c��H+��+c��H2C2O4��-c��C2O42-�� | |
| B�� | pH��ȵĢ�CH3COONa����C6H5ONa����Na2CO3����NaOH������Һ�����ʵ���Ũ�ȴ�С���٣��ڣ��ۣ��� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϵ���Һ�У�c��Cl-��+c��H+����c��NH4+��+c��OH-�� | |
| D�� | ��0.1 mol•L-1 NH4HSO4��Һ�еμ�NaOH����Һǡ�ó����ԣ�c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+�� |
���� A������KHC2O4��Һ�е������غ��жϣ�
B����CH3COONa����C6H5ONa����Na2CO3����ǿ�������Σ�����Խǿ����Ӧ������ӵ�ˮ��̶�Խ������ҺpHԽС���ݴ��ж�pH���ʱ���ߵ�Ũ�ȴ�С����������Ϊǿ����Һ����pH���ʱ��Ũ����С��
C���κ���Һ���������غ㣬���ݵ���غ������
D����Һ��ʾ���ԣ�c��H+��=c��OH-���������Һ�е���غ㡢�����غ��жϸ�����Ũ�ȴ�С��
��� �⣺A��0.1mol•L-1 KHC2O4��Һ�У����������غ�ɵã�c��OH-��=c��H+��+c��H2C2O4��-c��C2O42-������A��ȷ��
B����CH3COONa����C6H5ONa����Na2CO3����ǿ�������Σ�����Խǿ����Ӧ������ӵ�ˮ��̶�Խ������ҺpHԽС�����Ӧ������ӵ�ˮ��̶ȴ�СΪ��CH3COO-��C6H5O-��CO32-��pH���ʱ��ҺŨ�ȴ�СΪ��CH3COO-��C6H5O-��CO32-�������٣��ڣ��ۣ���NaOHΪǿ���pH���ʱ�������Ƶ�Ũ����С������������Һ�����ʵ���Ũ�ȴ�С���٣��ڣ��ۣ��ܣ���B��ȷ��
C�������£�pH=2��������pH=12�İ�ˮ�������ϵ���Һ�У����ݺ˵���غ�ɵã�c��Cl-��+c��H+��=c��NH4+��+c��OH-������C����
D�����������غ�ɵã�c��SO42-��=c��NH4+��+c��NH3•H2O������c��SO42-����c��NH4+����������ҺΪ���ԣ���c��H+��=c��OH-�������ݵ���غ�ɵã�c��H+��+c��Na+ ��+c��NH4+��=c��OH-��+2c��SO42-��������c��Na+ ��+c��NH4+��=2c��SO42-�������c��SO42-����c��NH4+����֪��c��Na+ ����c��SO42-����������Һ������Ũ�ȴ�СΪ��c��Na+����c��SO42-����c��NH4+����c��H+��=c��OH-������D��ȷ��
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Ӧ���������Ϊ���ؼ���ע�����յ���غ㡢�����غ㡢�����غ㡢�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷�����

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�| A�� | ������ | B�� | ����ƿ | C�� | Բ����ƿ | D�� | ��ͷ�ι� |
 �ձ�һ�ҹ�˾��ǰ�����������Ѿ��������ƻ���������һ�ֵ߸��Ե���������������̼���ϵ�˫̼�Ե�أ��ŵ�ԭ��ʾ����ͼ��ʾ��������ٶȱ���ͨ������ӵ�ؿ�20�����ŵ�ʱ��������ӦCn��PF6��+e-�TPF6-+nC��������ӦLiCn-e-�TLi++nC�����й�˵������ȷ���ǣ�������
�ձ�һ�ҹ�˾��ǰ�����������Ѿ��������ƻ���������һ�ֵ߸��Ե���������������̼���ϵ�˫̼�Ե�أ��ŵ�ԭ��ʾ����ͼ��ʾ��������ٶȱ���ͨ������ӵ�ؿ�20�����ŵ�ʱ��������ӦCn��PF6��+e-�TPF6-+nC��������ӦLiCn-e-�TLi++nC�����й�˵������ȷ���ǣ�������| A�� | a��Ϊ��صĸ��� | |
| B�� | A-ΪOH- | |
| C�� | ��س��ʱ������ӦΪ��LiCn+e-�TLi++nC | |
| D�� | ���ʱ����Һ��A-��b����a��Ǩ�� |
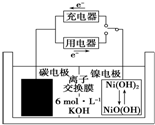 һ��̼�����ܹ������������������ε�أ���ͼ��ʾ����̼�缫���õ�صĵ������ҺΪ6mol•L-1��KOH��Һ������˵����ȷ���ǣ�������
һ��̼�����ܹ������������������ε�أ���ͼ��ʾ����̼�缫���õ�صĵ������ҺΪ6mol•L-1��KOH��Һ������˵����ȷ���ǣ�������| A�� | ���ʱ��������������Ӧ | |
| B�� | ���ʱ��̼�缫���Դ���������� | |
| C�� | �ŵ�ʱ̼�缫��ӦΪH2-2e-�T2H+ | |
| D�� | �ŵ�ʱ���缫��ӦΪNiO��OH��+H2O+e-�TNi��OH��2+OH- |
| A�� | �ƺͼصĺϽ��ڳ�������Һ�壬�����ڿ����ӷ�Ӧ�����Ƚ����� | |
| B�� | �Ȼ�����Һ�����ԣ������������������Ȼ�����Һ | |
| C�� | ̼������Һ�ʼ��ԣ������ȵĴ�����Һ��ȥ���������� | |
| D�� | �����£���ҵ���ô����ʯӢɰ�Ʋ�����˵�����������ǿ��̼�� |
�ٹ�����п��18mol/L������Һ��Ӧ
�ڹ����������������ĵ����ڴ������ں�һ�������³�ַ�Ӧ
�ۼ���������Ũ���������MnO2 ��Ӧ
�ܼ��������¹���ͭ��Ũ���ᷴӦ
�ݹ���ϡ�������״ʯ��ʯ��Ӧ��
| A�� | �ڢۢܢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢۢܢ� |
| A�� | $\frac{3a}{2B}$mol/L | B�� | $\frac{a}{27B}$mol/L | C�� | $\frac{a}{18B}$mol/L | D�� | $\frac{2a}{81B}$mol/L |
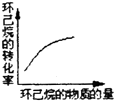
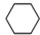 ��g��$?_{����}^{Pt-Sn/Al_{2}O_{3}}$
��g��$?_{����}^{Pt-Sn/Al_{2}O_{3}}$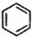 ��g��+3H2��g����H��0�����÷�Ӧ�ں��ݵ��ܱ������н��У������йظ÷�Ӧ��ͼ���ж���ȷ���ǣ�������
��g��+3H2��g����H��0�����÷�Ӧ�ں��ݵ��ܱ������н��У������йظ÷�Ӧ��ͼ���ж���ȷ���ǣ�������