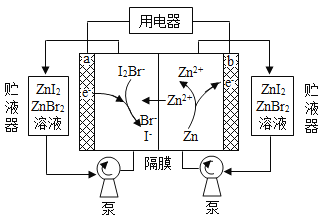
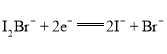��Ŀ����
����Ŀ����֪ijNaOH�����к���NaCl���ʣ�Ϊ�ⶨ������NaOH�������������������²���ʵ�飺
�� ����1.000 g��Ʒ����ˮ�����250 mL��Һ��
�� ȷ��ȡ25.00 mL������Һ����ƿ�У�
�� �μӼ��η�̪��Һ��
�� ��0.1000 mol/L �ı�����ζ����Σ�ÿ����������������¼���£�
�ζ���� | ����Һ�����mL�� | �����������Һ�������mL�� | |
�ζ�ǰ���� | �ζ������ | ||
1 | 25.00 | 0.50 | 20.60 |
2 | 25.00 | 6.00 | 26.00 |
3 | 25.00 | 1.10 | 21.00 |
��ش�
��1����_______�ζ��ܣ�������ʽ��������ʽ����ʢװ0.1000 mol/L�������Һ��
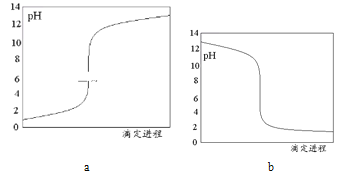
��2���жϵζ��յ㵽��ʱ������_________��
��3���õζ����̵ĵζ����������е�______��ѡ����a������b������
��4������������������ⶨ���ƫ�ߵ���__________��
a �ζ�ǰ������ˮ��ϴ��ƿ
b ������ƿʱ������ƿ����Һ����
c ���ڵζ������в�����������Һ������ƿ��
��5��ͨ�������֪���ռ���Ʒ�Ĵ���Ϊ______________��
���𰸡���ʽ ��ƿ�е���Һ�ɺ��Ϊ��ɫ b c 80.0%
��������
(1)���������ԣ�Ӧ�ü�ʽ�ζ���ʢװ0.10mol/L�������Һ��
(2)�μӼ��η�̪��NaOH��Һ�Ժ�ɫ�����ζ����յ�ʱ����Һ�����ԣ���Һ��Ϊ��ɫ��
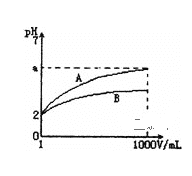
(3)��ƿ��ʢ�ŵ���NaOH��Һ�����Եζ�ʱ��Һ��pH��С��
(4)����c(NaOH)=![]() ������������
������������
(5)���ݵζ�ԭ�����з�����
(1)���������ԣ�Ӧ����ʽ�ζ���ʢװ0.10mol/L�������Һ��
(2)�μӼ��η�̪��NaOH��Һ�Ժ�ɫ�����ζ����յ�ʱ����Һ�����ԣ���Һ��Ϊ��ɫ����ζ��յ㵽��ʱ����������ƿ�е���Һ�ɺ��Ϊ��ɫ��
(3)��ƿ��ʢ�ŵ���NaOH��Һ�����Եζ�ʱ��Һ��pH��С����ͼ��֪b���߷��ϣ�
(4)����c(NaOH)=![]() �������
�������
a���ζ�ǰ������ˮ��ϴ��ƿ�������Ӱ�죬��a����
b��������ƿʱ������ƿ����Һ���������ƫ�ͣ���b����
c�����ڵζ������в�����������Һ������ƿ�⣬��Ҫ��������ᣬ���ƫ�ߣ���c��ȷ��
�ʴ�Ϊc��
(5)ͼ�����ݷ�����֪���ζ�ǰ�����ı���Һ����ֱ�Ϊ��20.60mL-0.50mL=20.10mL��26.00mL-6.00mL=20.00mL��21.00mL-1.10mL=19.90mL��ƽ���������=![]() =20.
=20.![]() 00mL���������ƺ��Ȼ������ʵ�����ͬ����250mL��Һ�����������������ʵ���=0.0200L��0.10mol/L��
00mL���������ƺ��Ȼ������ʵ�����ͬ����250mL��Һ�����������������ʵ���=0.0200L��0.10mol/L��![]() =0.020mol��
=0.020mol��
����������������=![]() ��100%=80%��
��100%=80%��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�