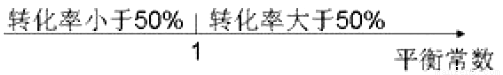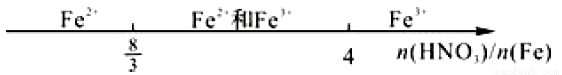��Ŀ����
������ЧӦ����ȫ���ע�Ļ�������֮һ��CO2��Ŀǰ�����к�����ߵ�һ���������壬CO2���ۺ������ǽ�����Ҽ���Դ�������Ч;����
��1���о�����CO2��H2�ڴ��������¿ɷ�����Ӧ����CH3OH����֪���ַ�Ӧ���Ȼ�ѧ����ʽ���£�
CH3OH(g)+ O2(g) = CO2(g)+2H2O(1) ��H1=akJ•mol-1
O2(g) = CO2(g)+2H2O(1) ��H1=akJ•mol-1
H2(g)+ O2(g) = H2O(1) ��H2=bkJ•mol-1
O2(g) = H2O(1) ��H2=bkJ•mol-1
H2O(g) = H2O(l) ��H3=ckJ•mol-1
�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=__________kJ•mol-1
CH3OH(g)+H2O(g) ��H=__________kJ•mol-1
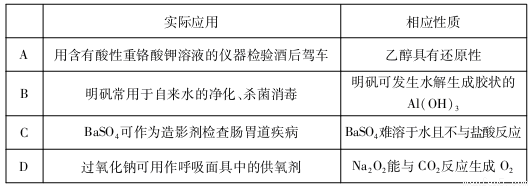
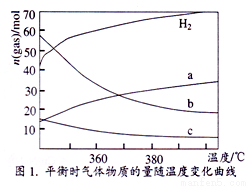
��2��CO2������Ҳ�ܺϳɵ�̼ϩ���� 2CO2(g)+6H2(g) C2H4(g)+4 H2O (g)����ͬ�¶���ƽ��ʱ��������̬���ʵ����ʵ�����ͼ1��ʾ������b��ʾ������Ϊ_______________ ��д��ѧʽ����
C2H4(g)+4 H2O (g)����ͬ�¶���ƽ��ʱ��������̬���ʵ����ʵ�����ͼ1��ʾ������b��ʾ������Ϊ_______________ ��д��ѧʽ����
��3��CO2��H2�ڴ���Cu/ZnO�����¿ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO��
��Ӧ A��CO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
��ӦB��CO2(g)+ H2(g) CO(g)+H2O(g)
CO(g)+H2O(g)
����CO2��H2��ʼͶ�ϱ�Ϊ1��3ʱ���¶ȶ�CO2ƽ��ת���ʼ��״���CO���ʵ�Ӱ����ͼ2��ʾ��
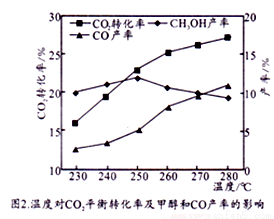
�� ��ͼ2��֪�¶�����CO�IJ�������������Ҫԭ�������__________________��
�� ��ͼ2��֪��ȡCH3OH�����˵��¶���________________�����д�ʩ���������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��__________________��
A.ʹ�ô��� B.������ϵѹǿ
C.����CO2��H2�ij�ʼͶ�ϱ� D.Ͷ�ϱȲ��������������䣬���ӷ�Ӧ���Ũ��
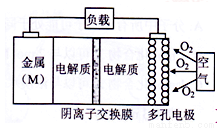
��4���ڴ�������ͨ��ʩ�ӵ�ѹ�ɽ��ܽ���ˮ�еĶ�����ֱ̼��ת��Ϊ�Ҵ����������Ҵ��ĵ缫��ӦʽΪ______________________________________________________��
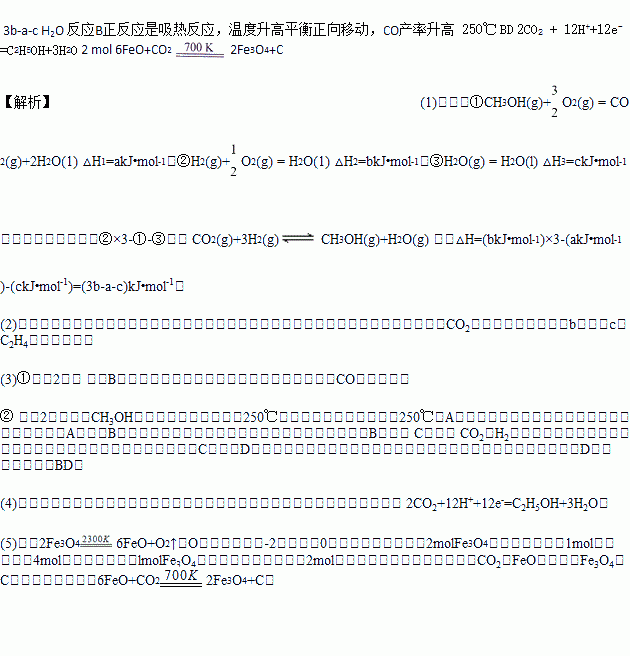
��5����CO2��ȡC��̫���ܹ�����ͼ3��ʾ�����ȷֽ�ϵͳ�������ķ�ӦΪ��2Fe3O4 6FeO+O2�� ��ÿ�ֽ�1mol Fe3O4ת�Ƶ��ӵ����ʵ���Ϊ_____________��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��
6FeO+O2�� ��ÿ�ֽ�1mol Fe3O4ת�Ƶ��ӵ����ʵ���Ϊ_____________��������ϵͳ��������Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�80��ʱ��NO2(g)+SO2(g) SO3(g)+ NO (g)�����¶��£��ڼס��ҡ��������������Һ��ݵ��ܱ������У�Ͷ��NO2��SO2����ʼŨ�����±���ʾ�����м�2min��ƽ��ʱ��NO2��ת����Ϊ50%������˵����ȷ����
SO3(g)+ NO (g)�����¶��£��ڼס��ҡ��������������Һ��ݵ��ܱ������У�Ͷ��NO2��SO2����ʼŨ�����±���ʾ�����м�2min��ƽ��ʱ��NO2��ת����Ϊ50%������˵����ȷ����
��ʼŨ�� | �� | �� | �� |
c(NO2)/mol • L-1 | 0.10 | 0.20 | 0.20 |
C(SO2)/ mol • L-1 | 0.10 | 0.10 | 0.20 |
A. �������еķ�Ӧ��ǰ2 min��ƽ������v(SO2)=0.05 mol • L-1• min-1
B. �ﵽƽ��ʱ��������������Ӧ���������������
C. �¶�����90�棬������Ӧƽ�ⳣ��Ϊ1.56����Ӧ�ġ�H>0
D. ������������ʼʱ�ij�0.10 mol•L-1 NO2��0.20mol•L-1 SO2���ﵽƽ��ʱc(NO)��ԭƽ����ͬ

 Ag+��aq��+Cl-��aq��
Ag+��aq��+Cl-��aq��