��Ŀ����
13��ij���½�һ��������һ�ֲ�Ʒ���ڡ����Ρ�����һ�����ᾧˮ���ò�Ʒ��������������鵵Ȼ�ľ�ķ��ϣ�ij��ѧ������ȤС��̽���ò�Ʒ����ɣ����������µ�ʵ�飺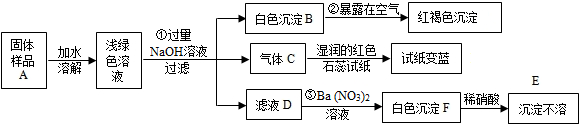
��1��д��ʵ������ȡC�Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��д�����б仯�Ļ�ѧ����ʽ��
��4Fe��OH��2+2H2O+O2�T4Fe��OH��3��
��Ba��NO3��2+FeSO4=BaSO4��+Fe��NO3��2��
��3������1.96g A�õ�E������Ϊ2.33g��C�ڱ�״���µ����Ϊ0.224L����A�Ļ�ѧʽΪ��NH4��2Fe��SO4��2•6H2O��
���� ����dz��ɫ��Һ�м�������������Ƶð�ɫ����B��B�ڿ����б�Ϊ���ɫ����ӦΪ��������������֪BΪFe��OH��2������C��ʹ��ɫʯ���������CΪ������D�м������ᱵ���ְ�ɫ���������������ᣬ��E��FΪBaSO4������D�к��У�����Ԫ���غ����֪dz��ɫ��Һ�к���SO42-��Fe2+��NH4+������1.96g A�õ�E������Ϊ2.33g����BaSO4�����ʵ���Ϊ0.01mol������dz��ɫ��Һ�к���SO42-�����ʵ���Ϊ0.01mol��C�ڱ�״���µ����Ϊ0.224L����NH4+�����ʵ���Ϊ0.01mol�����ݵ���غ��֪��dz��ɫ��Һ�к���Fe2+�����ʵ���Ϊ$\frac{0.01��2-0.01}{2}$mol=0.005mol������1.96g A�к��нᾧˮ�����ʵ���Ϊ$\frac{1.96-0.01��96-0.01��18-0.005��56}{18}$mol=0.03mol���ݴ˴��⣮
��� �⣺����dz��ɫ��Һ�м�������������Ƶð�ɫ����B��B�ڿ����б�Ϊ���ɫ����ӦΪ��������������֪BΪFe��OH��2������C��ʹ��ɫʯ���������CΪ������D�м������ᱵ���ְ�ɫ���������������ᣬ��E��FΪBaSO4������D�к��У�����Ԫ���غ����֪dz��ɫ��Һ�к���SO42-��Fe2+��NH4+������1.96g A�õ�E������Ϊ2.33g����BaSO4�����ʵ���Ϊ0.01mol������dz��ɫ��Һ�к���SO42-�����ʵ���Ϊ0.01mol��C�ڱ�״���µ����Ϊ0.224L����NH4+�����ʵ���Ϊ0.01mol�����ݵ���غ��֪��dz��ɫ��Һ�к���Fe2+�����ʵ���Ϊ$\frac{0.01��2-0.01}{2}$mol=0.005mol������1.96g A�к��нᾧˮ�����ʵ���Ϊ$\frac{1.96-0.01��96-0.01��18-0.005��56}{18}$mol=0.03mol��
��1��ʵ������ȡ�����Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2����Ӧ��Ϊ������������������������������Ӧ�Ļ�ѧ����ʽΪ4Fe��OH��2+2H2O+O2�T4Fe��OH��3����Ӧ��Ϊ�������������ᱵ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��
�ʴ�Ϊ��4Fe��OH��2+2H2O+O2�T4Fe��OH��3��Ba��NO3��2+FeSO4=BaSO4��+Fe��NO3��2��
��3���������������֪��A��n��SO42-����n��Fe2+����n��NH4+����n��H2O��=0.01��0.005��0.01��0.03=2��1����2��6������A�Ļ�ѧʽΪ��NH4��2Fe��SO4��2•6H2O��
�ʴ�Ϊ����NH4��2Fe��SO4��2•6H2O��
���� ������Ҫ�������ʵ���������ʣ��е��Ѷȣ����ʵ��ƶ��ǽ���ؼ�������ʱע��������ʵ�������ɫ�ı仯���������ƶϣ�����ʱע������Ԫ���غ�͵���غ㣮

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�| A�� | 1��6��9 | B�� | 1��2��3 | C�� | 1��3��3 | D�� | 1��3��6 |
| A�� | ����CO2ͨ�������ˮ�У�NH3•H2O+CO2=${NH}_{4}^{+}$+${HCO}_{3}^{-}$ | |
| B�� | �������ʵ�����NH4HSO3��NaOH��Һ��ϼ��ȣ�${HSO}_{3}^{-}$+OH-=${SO}_{3}^{2-}$+H2O | |
| C�� | �廯������Һ��ͨ������Cl2��Cl2+2Br-=2Cl-+Br2 | |
| D�� | ��1mol KOH����Һ�뺬2mol Ba��HCO3��2����Һ��ϣ�Ba2++${2HCO}_{3}^{-}$+2OH-=BaCO3��+${CO}_{3}^{2-}$+2H2O |

 ��
��