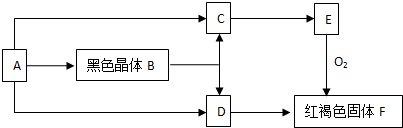
��Ŀ����
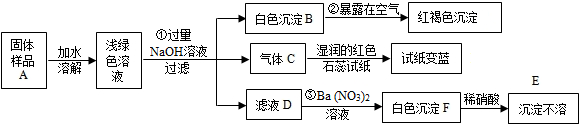
3��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��1����Ũ��������ʵ���Ũ��Ϊ11.8mol•L-1��
��2��ȡ�����������������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����BD��
A����Һ��Cl-����Ŀ B����Һ���ܶ� C�����ʵ����ʵ��� D����Һ��Ũ��
��3��ijѧ����������Ũ���������ˮ����450mL���ʵ���Ũ��Ϊ0.4mol/L��ϡ���ᣬ��ѧ����Ҫ��ȡ16.9mL����Ũ����������ƣ��������С�����һλ����������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬�������Ƶ�ϡ�������ʵ���Ũ�Ƚ�ƫ�ͣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ����������ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֲ���ˮ���̶��ߣ��������Ƶ�ϡ�������ʵ���Ũ�Ƚ�ƫ�ͣ�
��4��ȡ100mL 0.4mol•L-1��������100mL 0.1mol•L-1��AgNO3��Һ��ϣ���Ϻ������ɽ���Ϊ����Һ�����֮�ͣ���������Һ��Cl-�����ʵ���Ũ��Ϊ0.15mol•L-1��
���� ��1������c=$\frac{1000�Ѧ�}{M}$�����Ũ������HCl�����ʵ���Ũ�ȣ�
��2�����ݸ��������Ƿ�����Һ������й��жϣ�
��3��������Һϡ��ǰ�����ʵ����ʵ���������㣻����c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4��100mL 0.4mol•L-1��������HCl�����ʵ���n=CV=0.4mol/L��0.1L=0.04mol��100mL 0.1mol•L-1��AgNO3��Һ��AgNO3�����ʵ���n=CV=0.1mol/L��0.1L=0.01mol������Ϻ�����Ӧ��Ag++Cl-=AgCl�����������ߵ���������Cl-��Ũ�ȣ�
��� �⣺��1����������36.5%���ܶ�Ϊ1.18g/mL����������ʵ���Ũ��=$\frac{1000��1.18��36.5%}{36.5}$=11.8mol/L��
�ʴ�Ϊ��11.8��
��2��A����Һ��Cl-����Ŀ=nNA=CVNA����������Һ������йأ���A��ѡ��
B����Һ���ܶ�����Һ������أ���Bѡ��
C����Һ��HCl�����ʵ���n=CV����������Һ������йأ���C��ѡ��
D����Һ��Ũ��C=$\frac{1000�Ѧ�}{M}$������Һ������أ���Dѡ��
��ѡBD��
��3������ʵ������450mL����ƿ����Ӧѡ��500mL������ƿ�����ó�500mL����Һ��������Һϡ��ǰ�����ʵ����ʵ������䣬����ҪŨ��������ΪV����11.8mol/L��V=0.5L��0.400mol/L
���V=0.0169L=16.9mL��
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬�ᵼ����ȡ��Ũ��������ƫС���������Ƶ�ϡ�������ʵ���Ũ��ƫ�ͣ�ҡ�Ⱥ���Һ����ڿ̶��ߣ��������ģ��ֲ���ˮ���̶��ߣ��������Ƶ�ϡ�������ʵ���Ũ�Ƚ�ƫ�ͣ��ʴ�Ϊ��16.9��ƫ�ͣ�ƫ�ͣ�
��4��100mL 0.4mol•L-1��������HCl�����ʵ���n=CV=0.4mol/L��0.1L=0.04mol��100mL 0.1mol•L-1��AgNO3��Һ��AgNO3�����ʵ���n=CV=0.1mol/L��0.1L=0.01mol��
��������Ϻ�����Ӧ��Ag++Cl-=AgCl����֪��HCl�������ʷ�Ӧ����Һ�е�n��Cl-��=0.04mol-0.01mol=0.03mol���ʷ�Ӧ���Ũ��c��Cl-��=$\frac{0.03mol}{0.2L}$=0.15mol/L���ʴ�Ϊ��0.15mol/L��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ��㡢�������Լ���Һ��Ϻ�����Ũ�ȵļ��㣬���ڻ�������Ŀ���ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ������������ʱ������Ӧ�����ʵ�������Ӧ��������ѧƽ�ⷢ���ƶ� | |
| B�� | ������������ʱ�������¶ȣ���ѧƽ������ȷ�Ӧ�ķ����ƶ� | |
| C�� | ������������ʱ������ѹǿ����ѧ��Ӧ��������ѧƽ�ⷢ���ƶ� | |
| D�� | ������������ʱ��ʹ�ô�������ѧ��Ӧ���ʸı䣬��ѧƽ�ⲻ�ƶ� |
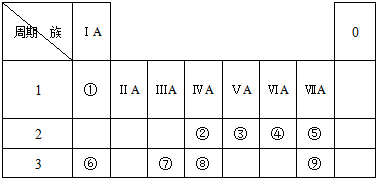
��1������C��
��2��9��Ԫ���зǽ�������ǿ����F��
��3���ܢޢߵ�ԭ�Ӱ뾶��С�����˳��ΪO��Al��Na��
��4���ڢۢ����ۺ����������������ǿ��˳����H2SiO3��H2CO3��HNO3��
��5��������ijЩԪ����ɵ��л�������
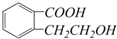 �������ʽ��C9H10O3�����������ŵ������ǣ��Ȼ������ǻ���
�������ʽ��C9H10O3�����������ŵ������ǣ��Ȼ������ǻ�����6��ij��ѧ��ȤС��Ϊ̽���ᵥ�ʡ�Br2��Fe3+��������ǿ�������������ʵ�飺
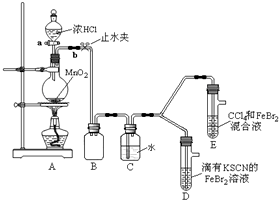
������ʵ��װ�ô���һ�����ԵIJ��㣬��ָ��ȱ��β������װ�ã�
���ø������װ�ý���ʵ�飬ʵ��������£�
| ʵ����� | ʵ������ | ���� |
| ����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��ƣ� | Dװ���У���Һ��� Eװ���У�ˮ����Һ��ƣ���CCl4�������Ա仯������ͨ��һ��ʱ���CCl4���Ϊ��ɫ | �ᵥ�ʡ�Br2��Fe3+����������ǿ������˳���ǣ� �ᵥ�ʣ���Cl2����Br2��Fe3+ |
| A�� | 0.9 mol H2O | B�� | 0.3 mol H2SO4 | C�� | 0.2 mol NH3 | D�� | 0.4 mol CH4 |
 Ӫ��ƽ�⡢��ѧʹ��ʳƷ���Ӽ������ڽ����������������
Ӫ��ƽ�⡢��ѧʹ��ʳƷ���Ӽ������ڽ����������������