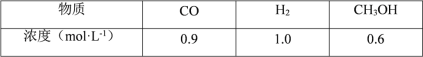��Ŀ����
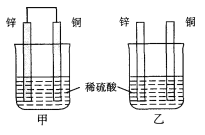
����Ŀ�������������������ܺ����Ա��㷺Ӧ�������ࡢ���ӡ������ȹ�ҵ���϶�ӰҺ������Ҫ��Na3Ag(S2O3)2��ʽ���ڣ�ʵ�����÷϶�ӰҺ�Ʊ�Ag�ľ���������ͼ��ʾ��
ע������ԭ��ʱ����Ag+ֱ����N2H4H2O��Ӧ���ڼ��ң����Բ��ü��백ˮ��ʹAg+�백�γ�[Ag(NH3)2]+������Ag+��Ũ�ȣ��Ӷ���Ӧ����Ag+������������ʹ��Ӧ�ܹ�ƽ�Ƚ��С�
�ش��������⣺
��1����������ʱ������������___(�ѧʽ����
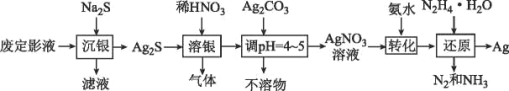
��2��N2H4H2O(ˮ���£�Ϊ��ɫ������״����Һ�壬����ǿ��ԭ�ԣ�ʵ�����Ʊ�ԭ��ΪNaClO+2NH3=N2H4H2O+NaCl�������õ���ʵ��װ����ͼ��ʾ��
�ٱ�ʵ�����ò�����װ����___��(����ĸ�����Լ�x��___(�ѧʽ����дһ�֣���
�ڼ���NaClO��ҺʱҪ�����μӣ�Ŀ����___��
�۰����������ҵķ�������װ�õ�����˳��Ϊ___(�������ӿ�Сд��ĸ����
��3��AgNO3����������ֽ⡣������������AgNO3��Һ����Ũ���ɻ��AgNO3���壬ʵ��װ����ͼ��ʾ��
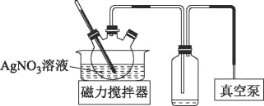
��ʹ����ձõ�Ŀ����___��
�ڲⶨAgNO3����Ĵ���(���ʲ����뷴Ӧ����ȡ2.00g�Ʊ���AgNO3���壬��ˮ�ܽ⣬���ݵ�100mL��ȷ��ȡ25.00mL��Һ���ữ����뼸��NH4Fe(SO4)2��Һ��ָʾ��������0.1000molL-1NH4SCN����Һ�ζ�������NH4SCN����Һ��ƽ�����Ϊ29.00mL���������AgNO3����������Ϊ___��
���𰸡�NO D CCl4 ˮ���»�ԭ�Ժ�ǿ����ֹNaClOŨ�ȹ��������� ehi(ih)abc(d) ʹ��ϵ�γɸ�ѹ��������ˮ���ڽϵ͵��¶���������ͬʱ��ֹAgNO3�ֽ� 98.6%
��������
(1)��������ͼ����������������Ag2S�м���ϡ���ᣬ����������ԭ��Ӧ�Ƕȷ�����
(2)�������Ʊ�N2H4H2O�ķ�Ӧ��֪����Ҫ�Ʊ���������ϵװ��ͼ����ϰ�����N2H4H2O���������ѡ��ʵ��װ�ý��з�����������������ˮ��
��N2H4H2O����ǿ��ԭ�ԣ�NaClO���������ԣ�
�����(2)�ķ���������������
(3)����������ṩ��Ϣ��AgNO3����������ֽ���з�����
��NH4SCN����Һ��ƽ�����Ϊ29.00mL����NH4SCN�����ʵ���Ϊ0.1000molL-1��0.029L=2.9��10-3mol������Ag++SCN-=AgSCN�������������������������ȷ������������
(1)��������ͼ����������������Ag2S�м���ϡ���ᣬAg2S�е���Ϊ-2�ۣ�������ͼ�̬�����л�ԭ�ԣ�������������ԣ����߷���������ԭ��Ӧ��Ag2S�е��ϼ�����ת��Ϊ���ʣ�ϡ������������ԭΪNO�����������������в���������ΪNO��
(2)�ٸ����Ʊ�N2H4H2O�ķ�ӦNaClO+2NH3=N2H4H2O+NaCl��֪����Ҫ�Ʊ�������ʵ�������Ȼ�狀��������ƹ��������ȡ��������Ӧѡ��װ��C������װ��A�з�Һ©���е�ҩƷΪNaClO����Aװ��Ϊ��ȡˮ���µ�װ�ã�װ��E��ȫƿ���ã�������������ˮ������ʹ��װ��D��Ҫʹ��װ��B����������β��ͬʱ��ֹ����������Bװ����Һ��ֲ㣬��ˮ���ϲ㣬x�Լ����²㣬˵��x�Լ��ܶȱ�ˮ����ˮ�����ܣ������ܽⰱ���������ѧ֪ʶ����x�Լ���ΪCCl4�ȣ�
��N2H4H2O����ǿ��ԭ�ԣ�NaClO���������ԣ���NaClO��Һ������쵼�¹������ɽ���Ӧ���ɵ�N2H4H2O�������������μ�NaClO��Һ�ܷ�ֹ���ɵ�N2H4H2O��������
�۽�Ϣ��еķ���������������ҵķ���װ�õ�����˳��Ϊehi(ih)abc(d)��
(3)�ٸ�������ṩ��Ϣ��AgNO3����������ֽ⣬ʵ��װ������ձÿ����γɸ�ѹ��������ˮ���ڽϵ͵��¶���������ͬʱ��ֹAgNO3�ֽ⣻
��NH4SCN����Һ��ƽ�����Ϊ29.00mL����NH4SCN�����ʵ���Ϊ0.1000molL1��0.029L=2.9��103mol�����������غ㣬��Ϸ�ӦAg++SCN=AgSCN����֪��������������Ϊ2.9��103mol��170g/mol��![]() =1.972g����������������������Ϊ
=1.972g����������������������Ϊ![]() ��100%=98.60%��
��100%=98.60%��

 �Ķ��쳵ϵ�д�
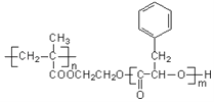
�Ķ��쳵ϵ�д�����Ŀ������ᣨ ����Ҫ�����㾫���ϡ�ʳƷ���Ӽ����л��ϳɵȷ��棬���ĺϳ�ԭ��Ϊ
����Ҫ�����㾫���ϡ�ʳƷ���Ӽ����л��ϳɵȷ��棬���ĺϳ�ԭ��Ϊ
![]()
 +CH3COOH
+CH3COOH
��Ҫ�Լ������������ʣ�
���� | ��Է� ������ | ��״ | �ܶ�/ ��g��cm-3�� | �۵�/�� | �е�/�� | �ܽ��/��g/100 mL�ܼ��� | ||
ˮ | �� | �� | ||||||
����ȩ | 106 | ��ɫ Һ�� | 1.044 | -26 | 178��179 | �� | �� | �� |
������ | 102 | ��ɫ Һ�� | 1.082 | -73 | 138��140 | ��Ӧ | �� | ���� |
����� | 148 | ��ɫ ��ĩ | 1.248 | 133��134 | 300 | �� | �� | �� |
�Լ�������
�Լ����� | ����ȩ | ������ | ��ˮ̼��� | 10%�������� | Ũ���� | ����̿ |
�Լ����� | 3.0 mL | 5.5 mL | 4��5 g | 40 mL | 25 mL | 1.0 g |
ʵ���������£�

��ش��������⣺
��1��ʵ�����Σ�����װʵ������ǰҪȷ����Ӧ����װ�ó������ԭ���ǣ�_______��
��2����3.0 mL����ȩ��5.5 mL��������4.00 g��ˮ̼������μ���250 mL������ƿ��ҡ�ȣ���ƿ�ײ��а�ɫ����״�������ɣ��ϲ�Һ����ɫ������Ӧ���ң��а���ð������û���װ�ú�ʼ���Ȼ��������Ȼ���ʱ���Ʒ�Ӧ����״̬�������¶Ȳ���̫�ߵ�ԭ��_____��
��3��ʵ���������������ƿ�м���40 mL 10%������������Һ��20 mL��ˮ���ɹ۲쵽ĸҺ��ϵ��Ϊ�����ˮ�ࡣ��װ�ø�Ϊˮ��������װ�ã���ʼ��������ʹ�����еı���ȩ��ˮ�����뿪ĸҺ����ʼ�ռ�������NaOH�������ǣ�__________��Bװ���еij��������ܵ������ǣ�_______��ˮ��������װ������Ҫ���ȵ�������____����װ����ţ���
��4�����������������м��ȣ��������ȶ�����ͨ����ƿ�е�Һ���£���ʼ���������������������͵κ�ֹͣ������1.0 g����̿��ɫ���ȹ��ˡ�����̿��ɫ��ԭ����______��
��5������25 mL��Ũ���ᣬ��ˮԡ���ձ����а�ɫ�������֡����ˣ��ñ�ˮϴ�ӣ������������������ص�m=0.35 g����÷�Ӧ�IJ���ԼΪ_____�������ȷ��0.1% ����
����Ŀ����25 ��ʱ��AgCl�İ�ɫ����Һ�У����μ����Ũ�ȵ�KI��Һ��Na2S��Һ���۲쵽���������ȳ��ֻ�ɫ���������ճ��ֺ�ɫ��������֪�й����ʵ��ܶȻ�Ksp(25 ��)���£�
AgCl | AgI | Ag2S | |
Ksp | 1.8��10��10 | 8.51��10��16 | 6.3��10��50 |
��������������ǣ� ��
A.����ת����ʵ�ʾ��dz����ܽ�ƽ����ƶ�
B.�ܽ��С�ij�������ת��Ϊ�ܽ�ȸ�С�ij���
C.AgCl�����ڵ����ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܽ�̶���ͬ
D.25 ��ʱ���ڱ���AgCl��AgI��Ag2S��Һ�У�����Ag����Ũ�Ȳ�ͬ