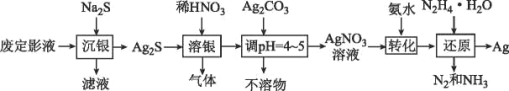
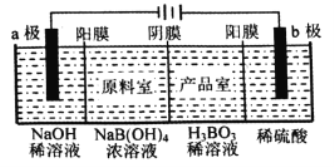
��Ŀ����
����Ŀ����25 ��ʱ��AgCl�İ�ɫ����Һ�У����μ����Ũ�ȵ�KI��Һ��Na2S��Һ���۲쵽���������ȳ��ֻ�ɫ���������ճ��ֺ�ɫ��������֪�й����ʵ��ܶȻ�Ksp(25 ��)���£�
AgCl | AgI | Ag2S | |
Ksp | 1.8��10��10 | 8.51��10��16 | 6.3��10��50 |
��������������ǣ� ��
A.����ת����ʵ�ʾ��dz����ܽ�ƽ����ƶ�
B.�ܽ��С�ij�������ת��Ϊ�ܽ�ȸ�С�ij���
C.AgCl�����ڵ����ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܽ�̶���ͬ
D.25 ��ʱ���ڱ���AgCl��AgI��Ag2S��Һ�У�����Ag����Ũ�Ȳ�ͬ
���𰸡�C
��������
A. ����ת����ʵ�ʾ�������������ת��Ϊ�����ܵ����ʣ������ڳ����ܽ�ƽ����ƶ�����A��ȷ��
B. ������ͬ���͵��������Σ�һ��������ܶȻ���ij�������ת�����ܶȻ�С�ij���������AgCl�İ�ɫ����Һ�м���KI��Һ��������AgI��������B��ȷ��
C. �����ܶȻ��dz�������������Ũ�ȴ���������Ũ��С����C����
D. ����AgCl��AgI��Ag2S��Һ��Ag+��Ũ�ȷֱ�Ϊ��![]() ��1��10-5mol/L��
��1��10-5mol/L��![]() ��3��10-8mol/L��
��3��10-8mol/L��![]() ��2��10-16mol/L��Ag+��Ũ�Ȳ�ͬ����D��ȷ��
��2��10-16mol/L��Ag+��Ũ�Ȳ�ͬ����D��ȷ��
��ĿҪ��ѡ�����ģ���ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��CO2�ڹ�ҵ������Ҫ����;����ش�
I.��CrO3��������CO2��������(C2H6)����ϩ(C2H4)�ķ�Ӧ��������:
��C2H6(g)![]() C2H4(g)+H2(g) ��H1��
C2H4(g)+H2(g) ��H1��
��3H2(g)+2CrO3(s) =3H2O(g)+Cr2O3(s) ��H2��
��Cr2O3(s)+3CO2(g)=3CO(g)+2CrO3(s) ��H3��
(1)��ӦC2H6(g)+CO2(g)![]() C2H4(g)+CO(g)+H2O(g)�ķ�Ӧ����H=____________ (�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
C2H4(g)+CO(g)+H2O(g)�ķ�Ӧ����H=____________ (�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
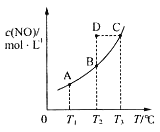
(2)��2L�ĺ����ܱ������г���0.1molC2H6(g)��0.1molCO2(g)������(1)�з�Ӧ��C2H6(g)��ƽ��ת����[a(C2H6)]���¶�(T)�Ĺ�ϵ��ͼ��ʾ��
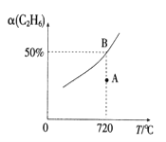
������Ӧ�¶��£�A��ķ�Ӧ��v(��)____v(��)(����>������<������=��)��
���������Ǹ���Ӧ�ķ�����Ϊ�����(C2H6)�����˽������Pʱ����ϵ�з�������⣬���ɲ�ȡ�Ĵ�ʩ��___________(��дһ��)��
��.�����Ϊ2L���ܱ������У��������»�ѧ��Ӧ��CO2(g)+H2(g)![]() CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���ʾ��
CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���ʾ��
T/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
(3)�÷�ӦΪ_________��Ӧ(��������������������)��ԭ��Ϊ_________________��
(4)830���£����������зֱ����2molH2��2molCO2��10s��ﵽƽ�⣬�����ʱ����v(H2)=________��ת������(CO2)=___________��