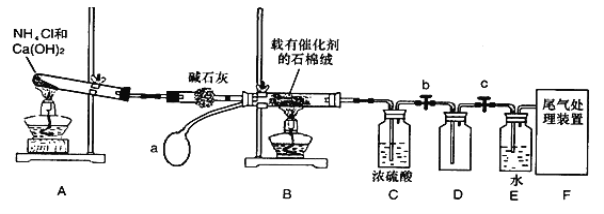
��Ŀ����
����Ŀ����֪��A��ʯ���ѽ�������Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־���������л���A��G֮���ת����ϵ��
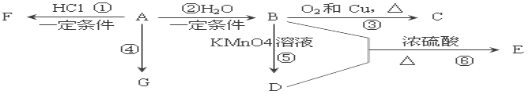
��ش��������⣺
(1)A�Ĺ����ŵ�������____________��C�Ľṹ��ʽ��__________��
(2)E��һ�־�����ζ��Һ�壬��B+D��E�ķ�Ӧ������_______���÷�Ӧ����ʽΪ_________��
(3)G��һ�ָ߷��ӻ������ṹ��ʽ��__________��
(4)�����У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ��������F����Ӧ��������д����A��F�Ļ�ѧ��Ӧ����ʽ_____________��
���𰸡�̼̼˫�� CH3CHO ������Ӧ(��ȡ����Ӧ) CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O ![]() CH2=CH2+HCl
CH2=CH2+HCl![]() CH3CH2Cl
CH3CH2Cl
��������
A��ʯ���ѽ�������Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��A����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����B����B���Ҵ����Ҵ���������ͭ�����������´���Ӧ����C��C����ȩ��B�Ҵ��ڸ����������������D��D�����ᣬ�Ҵ���������Ũ���������·�Ӧ������������E��G��һ�ָ߷��ӻ������A��ϩ�Ӿ۶���Ϊ����ϩ![]() ��FΪA��ϩ���Ȼ���ӳɶ���Ϊ�����顣
��FΪA��ϩ���Ȼ���ӳɶ���Ϊ�����顣
��1���ɷ�����֪AΪ��ϩ���ṹ��ʽΪ��CH2=CH2������C=C˫����B���Ҵ����Ҵ���������ͭ�����������´�������Ӧ����C��C����ȩ��C�Ľṹ��ʽΪCH3CHO���ʴ�Ϊ��̼̼˫����CH3CHO��
��2��E��һ�־�����ζ��Һ�壬Ϊ����������B���Ҵ���B�Ҵ��ڸ����������������D��2C2H5OH+2KMnO4+5H2SO4=K2SO4+2MnSO4+2CH3COOH+7H2O��D�����ᣬ�Ҵ���������Ũ���������·���������ӦCH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��������Ӧ(��ȡ����Ӧ) ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3����A��ϩ�Ӿ۶���Ϊ����ϩ������![]() ��G�Ľṹ��ʽΪ��
��G�Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4��FΪA��ϩ���Ȼ��ⷴӦ�IJ����ϩ���Ȼ�����һ�������·����ӳɷ�Ӧ���������飬����ʽΪ��CH2=CH2+HCl![]() CH3CH2Cl��������е�͡��ӷ���ʹ���˲�λƤ�������¶���Ȼ�½����ܼ�����Ա��ʹ�У��ʴ�Ϊ��CH2=CH2+HCl
CH3CH2Cl��������е�͡��ӷ���ʹ���˲�λƤ�������¶���Ȼ�½����ܼ�����Ա��ʹ�У��ʴ�Ϊ��CH2=CH2+HCl![]() CH3CH2Cl��
CH3CH2Cl��

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�����Ŀ��һ���¶��£���ij2 L�����ܱ������м�������������ͭ��ͨ��0.1 mol ˮ(g)���������·�Ӧ��2H2O(g)![]() 2H2(g)��O2(g)����H����484 kJ��mol��1����ͬʱ�����O2�����ʵ������±���
2H2(g)��O2(g)����H����484 kJ��mol��1����ͬʱ�����O2�����ʵ������±���
ʱ��/min | 20 | 40 | 60 | 80 |
n(O2)/mol | 0.0010 | 0.0016 | 0.0020 | 0.0020 |
����˵������ȷ����(����)
A.ǰ20 min�ڵ�ƽ����Ӧ����v(H2O)��5��10��5mol��L��1��min��1
B.�ﵽƽ��ʱ����Ҫ��������յ�����Ϊ0.968 kJ
C.����ˮ��Ũ�ȣ����Ըı����Ӧ����
D.ʹ��������������ͭ����������ƽ��ʱ�������������