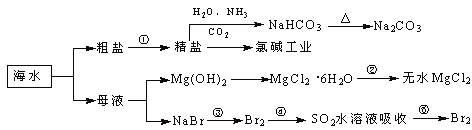
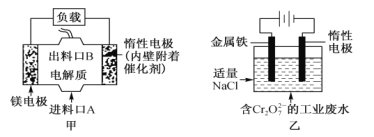
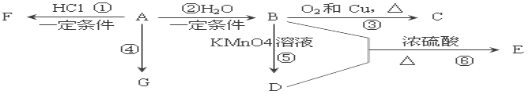
��Ŀ����
����Ŀ����.��֪����ӦaA(g)��bB(g)cC(g)��ij�¶��£���2L���ܱ�������Ͷ��һ������A��B��������������ʵ���Ũ����ʱ��仯��������ͼ��ʾ��
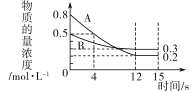
��1������4sʱ�䣬v(C)��0.05mol��L��1��s��1����4sʱ����C�����ʵ���Ϊ___________________���÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
��2����12sʱ�䣬v(A)��___________,v(C)��___________���÷�Ӧ12sʱ___________�ﵽ��ѧƽ�⣨���ǡ�����
��.��3������˵������֤��H2(g)��I2(g)2HI(g)�Ѵ�ƽ��״̬����________(�����)��
A����λʱ��������nmolH2��ͬʱ������nmolHI
B��һ��H��H�����ѵ�ͬʱ������H��I������
C���¶Ⱥ����һ��ʱ�����������ɫ���ٱ仯
D����Ӧ����v(H2)��v(I2)��![]() v(HI)
v(HI)
���𰸡�0.4mol 3A(g)��B(g)2C(g) 0.05mol/(L![]() s) 0.03mol/(L
s) 0.03mol/(L![]() s) �� BC
s) �� BC
��������
����ͼ���֪��A��BΪ��Ӧ���A�ij�ʼ���ʵ���Ũ��Ϊ0.8mol/L��B�ij�ʼŨ��Ϊ0.5mol/L����Ӧ���е�12sʱ�ﵽƽ�⣬��ʱA��ƽ��Ũ��Ϊ0.2mol/L��B��ƽ��Ũ��Ϊ0.3mol/L����A��B�仯��Ũ�ȷֱ�Ϊ0.6mol/L��0.2mol/L����֪a:b=3:1��
��1������4sʱ�䣬v(C)��0.05mol��L��1��s��1����4sʱ����C�����ʵ���Ϊ0.05mol��L��1��s��1![]() 4s
4s![]() 2L=0.4mol����ͼ��֪��4s��A�ı仯Ũ��Ϊ0.3mol/L��C�ı仯Ũ��Ϊ0.2mol/L����a:c=3:2������a:b=3:1����a:b:c=3:1:2���ʸ÷�Ӧ�Ļ�ѧ����ʽΪ3A(g)��B(g)2C(g)��
2L=0.4mol����ͼ��֪��4s��A�ı仯Ũ��Ϊ0.3mol/L��C�ı仯Ũ��Ϊ0.2mol/L����a:c=3:2������a:b=3:1����a:b:c=3:1:2���ʸ÷�Ӧ�Ļ�ѧ����ʽΪ3A(g)��B(g)2C(g)��
��2���ӷ�Ӧ��ʼ��12sʱ��A��Ũ�ȱ仯����c=0.8mol/L-0.2mol/L=0.6mol/L��ʱ��Ϊ12s����v��A��=![]() ����v(A)��v(C)��3:2����
����v(A)��v(C)��3:2����![]() ����ͼ��֪12s������Ũ�Ȳ��ٸı䣬��Ӧ�ﵽƽ��״̬��
����ͼ��֪12s������Ũ�Ȳ��ٸı䣬��Ӧ�ﵽƽ��״̬��
��3��A.��λʱ��������n mol H2��ͬʱ������n mol HI������֮�Ȳ��������ʵ���֮�ȣ�A����
B.һ��H-H�����ѵ�Ч������H-I���γɵ�ͬʱ������H-I�����ѣ�˵�����淴Ӧ������ȣ�B��ȷ��
C.���������ɫ���ٱ仯��˵����������Ũ�Ȳ��䣬���淴Ӧ������ȣ�C��ȷ��
D.��Ӧ����v��H2��=v��I2��=![]() v��HI����δ����������Ĺ�ϵ��D����
v��HI����δ����������Ĺ�ϵ��D����
��ѡBC��