题目内容
【题目】(1)室温下,2g苯(C6H6)完全燃烧生成液态水和CO2,放出83.6kJ的热量,写出1molC6H6完全燃烧的热化学方程式:______。
(2)已知:Fe2O3(s)+![]() C(s)=
C(s)=![]() CO2(g)+2Fe(s)△H=+akJmol-1;C(s)+O2(g)=CO2(g)△H=-bkJmol-1,则2Fe(s)+
CO2(g)+2Fe(s)△H=+akJmol-1;C(s)+O2(g)=CO2(g)△H=-bkJmol-1,则2Fe(s)+![]() O2(g)=Fe2O3(s)的△H=________。
O2(g)=Fe2O3(s)的△H=________。
(3)已知几种化学键的键能如表所示:
化学键 | Cl—Cl | F—F | Cl—F |
键能/ kJ·mol—1 | 242 | 159 | 172 |
则反应Cl2(g)+3F2(g)![]() 2ClF3(g)的△H=_____________ kJ·mol-1。
2ClF3(g)的△H=_____________ kJ·mol-1。
(4)如图是乙烷、二甲醚燃烧过程中的能量变化图。
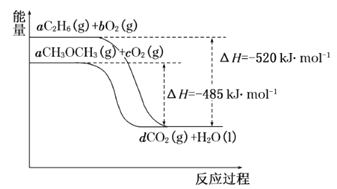
请回答下列问题:
①乙烷的燃烧热ΔH=_______kJ·mol-1。
②根据题图写出二甲醚完全燃烧时的热化学方程式__________。
【答案】C6H6(l)+![]() O2(g)=6CO2(g)+6H2O(l)△H=-3260.4kJ/mol (-
O2(g)=6CO2(g)+6H2O(l)△H=-3260.4kJ/mol (-![]() b-a)kJmol-1或-(
b-a)kJmol-1或-(![]() b+a)kJmol-1 -313 -1560 CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l) ΔH=-1455 kJ/mol
b+a)kJmol-1 -313 -1560 CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l) ΔH=-1455 kJ/mol
【解析】
(1)2g液态苯(C6H6)物质的量=![]() =
=![]() mol;完全燃烧生成液态水和CO2,放出83.6kJ的热量,1mol苯燃烧放热=83.6kJ×39mol=3260.4kJ;反应的热化学方程式为:C6H6(l)+
mol;完全燃烧生成液态水和CO2,放出83.6kJ的热量,1mol苯燃烧放热=83.6kJ×39mol=3260.4kJ;反应的热化学方程式为:C6H6(l)+![]() O2(g)=6CO2(g)+6H2O(l)△H=-3260.4kJ/mol;
O2(g)=6CO2(g)+6H2O(l)△H=-3260.4kJ/mol;
(2)①Fe2O3(s)+![]() C(s)=
C(s)=![]() CO2(g)+2Fe(s)△H=+akJmol-1
CO2(g)+2Fe(s)△H=+akJmol-1
②C(s)+O2(g)=CO2(g)△H=-bkJmol-1
根据盖斯定律,则2Fe(s)+![]() O2(g)=Fe2O3(s)可以根据
O2(g)=Fe2O3(s)可以根据![]() ×②-①得到,因此2Fe(s)+
×②-①得到,因此2Fe(s)+![]() O2(g)=Fe2O3(s)的△H=-
O2(g)=Fe2O3(s)的△H=-![]() b-akJmol-1或-(
b-akJmol-1或-(![]() b+a)kJmol-1;
b+a)kJmol-1;
(3)反应焓变△H=反应物总键能-生成物总键能,反应Cl2(g)+3F2(g)![]() 2ClF3(g)的△H=242kJ/mol+3×159kJ/mol-2×3×172kJ/mol=-313kJ/mol;
2ClF3(g)的△H=242kJ/mol+3×159kJ/mol-2×3×172kJ/mol=-313kJ/mol;
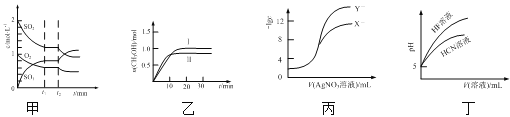
(4)①依据原子守恒分析可知氢原子守恒,6a=2,a=![]() ,则根据图象分析可知
,则根据图象分析可知![]() mol乙烷完全燃烧放热520kJ,所以1mol乙烷完全燃烧放热为520kJ×3=1560kJ,则乙烷的燃烧热△H=-1560kJ·mol-1;
mol乙烷完全燃烧放热520kJ,所以1mol乙烷完全燃烧放热为520kJ×3=1560kJ,则乙烷的燃烧热△H=-1560kJ·mol-1;
②根据图象分析可知![]() mol二甲醚完全燃烧放热485kJ,则1mol二甲醚完全燃烧放热=485kJ×3=1455kJ,反应的热化学方程式为:CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l)△H=-1455 kJmol-1。
mol二甲醚完全燃烧放热485kJ,则1mol二甲醚完全燃烧放热=485kJ×3=1455kJ,反应的热化学方程式为:CH3OCH3(g)+3O2(g)=2CO2(g)+3H2O(l)△H=-1455 kJmol-1。

 字词句段篇系列答案
字词句段篇系列答案【题目】(1)参考合成反应CO(g)+2H2(g)![]() CH3OH(g)的平衡常数,回答下列问题:
CH3OH(g)的平衡常数,回答下列问题:
温度/℃ | 0 | 50 | 100 | 200 | 300 | 400 |
平衡常数 | 667 | 100 | 13 | 1.9×10-2 | 2.4×10-4 | 1×10-5 |
①该反应正反应是___________(填“放热”或“吸热”)反应;
②在T℃时,1L密闭容器中,投入0.1molCO和0.2molH2,达到平衡时,CO转化率为50%,则T=__________℃。
(2)CH3OH也可由CO2和H2合成。在体积为1L的密闭容器中,充入lmolCO2和3molH2,一定条件下反应:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ΔH=-49.0kJ/mol,测得CO2和CH3OH(g)浓度随时间变化如图所示。
CH3OH(g)+H2O(g) ΔH=-49.0kJ/mol,测得CO2和CH3OH(g)浓度随时间变化如图所示。
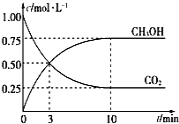
①该反应的平衡常数表达式为K=________;从反应开始到10min,v(H2)=______mol·L-1·min-1;
②下列情况能说明该反应一定达到平衡状态的是___________(填字母)
A.v(CO2)消耗=v(CH3OH)生成
B.气体的密度不再随时间改变
C.CO2和CH3OH的浓度之比不再随时间改变
D.气体的平均相对分子质量不再随时间改变
③为了加快化学反应速率且使体系中气体的物质的量增大,只改变下列某一条件,可采取的措施有___________ (填字母)
A.升高温度 B.缩小容器体积 C.再充入CO2气体 D.使用合适的催化剂
④相同温度下,在另一个容积为1 L的密闭容器中充入2mol CH3OH(g)和2molH2O(g),达到平衡时CO2的浓度____________(填“>”、“<”或“=”)0.25mol·L-1。