��Ŀ����
��Na+Ũ��Ϊ0.5mol/L��ij������Һ�У������ܺ����±��е����������ӣ�
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�м�������ϡHCl | ������ɫ�������ų�0.56L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4g |
| �� | �ڢ����Һ�еμ�BaC12��Һ | ���������� |
��1��ʵ��I��ȷ��һ�������ڵ��������� ��
��2��ʵ��I�����ɳ��������ӷ���ʽΪ ��
��3��ͨ��ʵ��I����ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ��
�����ڵ������0��������ȷ���Ƿ���ڵ������������
| ������ | NO3- | CO32- | SiO32- | SO42- |
| c/mol��L-1 | | | | |
��1��Ag+��Mg2+��Ba2+��2�֣���2��SiO32-+2H+ ��H2SiO3������SiO32-+2H++H2O��H4SiO4������2�֣�
��3����ÿ��1�֣�
��4�����ڣ�����СŨ��Ϊ0.8mol/L��2�֣������� NO3- CO32- SiO32- SO42- c/mol/L �� 0.25 0.4 0
�������������������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������档��ʵ����֪������Һ��һ������CO32-�������ᷴӦ����CO2�����ʵ�����0.56L��22.4L/mol��0.025mol�������̼ԭ���غ��֪��CO32����Ũ����Ũ��Ϊ��0.025mol��0.1L��0.25mol/L����Һ�к���CO32������һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+��H2SiO3��������ʵ����֪��2.4g�ǹ����Ƕ������裬�����ʵ�����2.4g��60g/mol��0.04mol������ݹ�ԭ���غ��֪SiO32-��Ũ��Ϊ0.04mol��0.1L��0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-����2��0.25mol/L+2��0.4mol/L��1.3mol/L��0.5mol/L�������Һ��һ������K+�����ڲ���ȷ��NO3-�Ƿ���ڣ�����K��Ũ������Ϊ0.8mol/L��
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
��2��ʵ��������ɳ��������ӷ���ʽΪ��SiO32-+2H+��H2SiO3����
��3��ͨ���������������֪��
��4�����ݵ���غ�2c��CO32-��+2c��SiO32-����2��0.25mol/L+2��0.4mol/L��1.3mol/L��0.5mol/L�������Һ��һ������K+�����ڲ���ȷ��NO3-�Ƿ���ڣ�����K��Ũ������Ϊ0.8mol/L�������� NO3- CO32- SiO32- SO42- c/mol/L �� 0.25 0.4 0
���㣺�������ӹ����Լ����Ӽ�����й��жϡ�����

 ��У����ϵ�д�
��У����ϵ�д���������͵�������(NOx)�Դ�����Ⱦ�������أ��о�����������Ⱦ�ķ����ǻ�ѧ�����ߵ���Ҫ���⣬Ŀǰ�кܶ��ַ�������������Ⱦ��
��1�������ü������ԭNOx�ķ�������NOx����Ӧ���£�
CH4(g)+4NO2(g)�� 4NO(g)+CO2(g)+2H2O(g)����H�� ��574 kJ��mol��1
CH4(g)+4NO(g)�� 2N2(g)+CO2(g)+2H2O(g)����H�� ��1160 kJ��mol��1
��CH4(g)+2NO2(g)�� N2(g)+CO2(g)+2H2O(g)����H�� ��
��2����������β���ķ���֮һ�����������ϰ�װ��ת�������������·�Ӧ��2NO(g)+2CO(g) N2(g)+2CO2(g)����H��0��
N2(g)+2CO2(g)����H��0��
����һ���¶��£���2molNO��1molCO����1L�̶��ݻ��������У�15���Ӻ�ﵽƽ�⣬��Ӧ�����и����ʵ�Ũ�ȱ仯��ͼ����ʾ����ƽ�ⳣ��K= (С�������3λ)��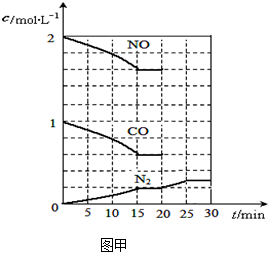
���������¶Ȳ��䣬20minʱ���������г���CO����0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
���������¶Ȳ��䣬20minʱ��ԭƽ�������г���CO��N2��0.6mol��ƽ�⽫ �ƶ�(����������ҡ�����)��
��20minʱ�����ı䷴Ӧ����������N2Ũ�ȷ�����ͼ��ʾ�ı仯����ı������������
(����ĸ)��
| A��������� | B�������¶� | C����С������� | D������CO2���� |