��Ŀ����
4����������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
������������SO2����������SO3��2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g��
ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ1��ʾ������ͼʾ�ش��������⣺
��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{{c}^{3}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$��
�ڽ�2.0mol SO2��1.0mol O2����10L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa���÷�Ӧ��ƽ�ⳣ������800��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��=K��B�������������������=������
����֪�������ȼ����Ϊ296KJ•mol-1��1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ•mol-1��������S��s������3molSO3��g���ġ�H=-1185kJ•mol-1��
��1����CH4����ԭNOx�������������������Ⱦ�����磺
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ•mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H=-1160kJ•mol-1
���ñ�״����4.48L CH4��ԭNO2��N2����������ת�Ƶĵ�������Ϊ1.6NA�������ӵ�������ֵ��NA��ʾ�����ų�������Ϊ��173.4kJ��
��2�������裨Si3N4����һ�������մɲ��ϣ�������ʯӢ�뽹̿�ڸ��µĵ������У�ͨ�����·�Ӧ�Ƶã�
3SiO2��s��+6C��s��+2N2��g��$\frac{\underline{\;����\;}}{\;}$Si3N4��s��+6CO��g��
�ﵽƽ��ı�ijһ������������ı�N2��CO����������Ӧ���ʦ���ʱ��t�Ĺ�ϵ��ͼ2��ͼ��t4ʱ����ƽ���ƶ�����������������ѹǿ��ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ����t3-t4��
���� I���ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{{c}^{3}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$��
�ڷ�Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa����ͼ��֪��SO2��ƽ��ת���ʦ�=0.80����������ʽ��ʾ��ƽ��ʱ��Ӧ��������ֵ����ʵ������ټ���ƽ��ʱ��Ӧ��������ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ���㣻
�ۻ�ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣻
�ܸ��ݸ�˹���ɼ��㷴Ӧ�ȣ�
II����1����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ•mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H=-1160kJ•mol-1
��Ӧ$\frac{��+��}{2}$��֪CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g����1molCH4��Ӧת��8mol���ӣ����ݸ�˹���ɼ����ʵ����뷴Ӧ�ȵ���ֵ��ϵ����ų���������
��2������ͼ֪��t2ʱ�ı����������淴Ӧ���ʶ�������ƽ�������ƶ����ı������Ϊ�¶ȣ�
ͼ��t4ʱ���淴Ӧ���ʶ�����ƽ�������ƶ����ı������������ѹǿ��
t6ʱ���淴Ӧ���ʶ�������Ȼ��ȣ�ƽ�ⲻ�ƶ����ı�������Ǵ�����ҪʹCO��Ũ�������ƽ����������Ӧ�����ƶ��������淴Ӧ�����ƶ�ʱ��
��� �⣺I���ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{{c}^{3}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$���ʴ�Ϊ��K=$\frac{{c}^{3}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$��
����ͼ��֪����ϵ��ѹǿΪ0.10MPaʱSO2��ƽ��ת����Ϊ0.8����
2SO2��g��+O2��g��$\frac{\underline{����}}{��}$2SO3��g��
��ʼ��2.0mol 1.0mol 0
ת����1.6mol 0.8mol 1.6mol
ƽ�⣺0.4mol 0.2mol 1.6mol
��ƽ��ʱ��c��SO2��=0.04mol/L��c��O2��=0.02mol/L��c��SO3��=0.16mol/L��
K=$\frac{{c}^{3}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$=$\frac{0.16��0.16}{��0.04��^{2}��0.02}$=800��
�ʴ�Ϊ��800��
�ۻ�ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬A��B���¶���ͬ������ƽ�ⳣ��K��A��=K��B�����ʴ�Ϊ��=��
�ܢ�S��s��+O2��g��=SO2��g����H1=-296 KJ•mol-1����SO2��g��+$\frac{1}{2}$O2��g��=SO3��g����H2=-99 kJ•mol-1��
�����ø�˹���ɽ��١�3+�ڡ�3�ɵ�3 S��s��+$\frac{9}{2}$O2��g��=3SO3��g����H3=3������H1+��H2��=-1185 kJ•mol-1��
�ʴ�Ϊ��-1185��
II����1����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ•mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H=-1160kJ•mol-1
��Ӧ$\frac{��+��}{2}$��֪CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g����1molCH4��Ӧת��8mol���ӣ�4.48L��������ʵ�����0.2mol��0.2mol���鷢��������Ӧʱת�Ƶ������ʵ�����1.6mol��ת�Ƶ�����Ϊ1.6NA��
��Ӧ$\frac{��+��}{2}$��֪CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g����H=-867kJ/mol����״����4.48LCH4�����ʵ���Ϊ0.2mol����ų�������Ϊ0.2mol��867kJ=173.4kJ��
�ʴ�Ϊ��1.6NA��173.4��
��2������ͼ֪��t2ʱ�ı����������淴Ӧ���ʶ�������ƽ�������ƶ����ı������Ϊ�¶ȣ�
ͼ��t4ʱ���淴Ӧ���ʶ�����ƽ�������ƶ����÷�Ӧ�Ƿ�Ӧǰ�������������Ŀ��淴Ӧ�����Ըı������������ѹǿ��
t6ʱ���淴Ӧ���ʶ�������Ȼ��ȣ�ƽ�ⲻ�ƶ����ı�������Ǵ�����ҪʹCO��Ũ�������ƽ����������Ӧ�����ƶ��������淴Ӧ�����ƶ�ʱ����ƽ��������CO�ĺ�����ߵ�һ��ʱ����t3-t4��
�ʴ�Ϊ������ѹǿ��t3-t4��
���� ���⿼�黯ѧƽ����㡢��ѧƽ���ƶ�Ӱ�����ء���˹�����йؼ����֪ʶ�㣬���ؿ���ѧ�����������������ѵ��Ǹ��ݸı�����ʱ���淴Ӧ���ʱ仯����Դ�С�жϷ�Ӧ������Ŀ�Ѷ��еȣ�

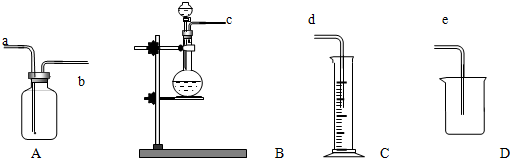
 ����������ϵ�д�
����������ϵ�д�| A�� | ��Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| B�� | ����ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� | |
| C�� | ������Ȼ�̼��Һ������ȡ�ķ�����������Ȼ�̼ | |
| D�� | ����ϡ����ʱ�������ձ���ע����������ˮ���ٻ���ע��Ũ���ᣬ���ò��������Ͻ��� |
| A�� | ���ԭ������ | B�� | Ԫ������ | C�� | ԭ������ | D�� | ͬλ������ |
| A�� | �����Һ���ȼ������ᱵ��Һ�а�ɫ�������ټ���ϡ���ᣬ��ɫ��������ʧ������ȷ�ϴ���Һ�к���SO42- | |
| B�� | �����Һ�м���NaOH��Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ����������ȷ�ϴ���Һ�к���NH4+ | |
| C�� | �����Һ���ȼ�����ˮ���ٵ���KSCN��Һ����Һ��죬����ȷ�ϴ���Һ�к���Fe2+ | |
| D�� | �ò�����պȡ����Һ���ھƾ��ƻ��������գ�����ʻ�ɫ������ȷ�ϴ���Һ�к���Na+ |

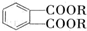 ������DMP����һ�ֳ��õ������ܻ�����������������������ܶ�Ϊ97����ҵ������DMP��������ͼ��ʾ��
������DMP����һ�ֳ��õ������ܻ�����������������������ܶ�Ϊ97����ҵ������DMP��������ͼ��ʾ��
 ������1��2-���ױ������ڶ��ױ�����C�й����ŵ�����Ϊȩ����DMP�ķ���ʽΪC10H10O4��
������1��2-���ױ������ڶ��ױ�����C�й����ŵ�����Ϊȩ����DMP�ķ���ʽΪC10H10O4�� ��
��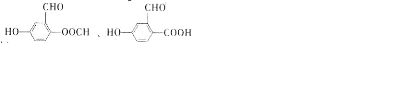 ��
�� �Ʊ�DMP����һ��;����
�Ʊ�DMP����һ��;����