题目内容
【题目】电解质溶液导电的本质是阴阳离子在电场作用下迁移,通过实验探究同一溶液中不同离子的迁移差异。将pH试纸用不同浓度Na2SO4的溶液充分浸湿,进行如下实验:
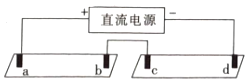
a,b,c,d均是石墨电极,电极间距4cm,电极电流0.20mA。实验现象:
时间 | 试纸Ⅰ | 试纸Ⅱ |
1min | a极试纸附近变红,b极附近试纸变蓝 | c极附近试纸变红,d极附近变蓝 |
10min | 红色区和蓝色区不断向中间扩展,相遇时红色区约2.7cm,蓝色区约1.3cm | 两极颜色范围扩大不明显,试纸大部分仍为黄色 |
对实验现象解释及预测不合理的是( )
A.b、d两极附近变蓝的原因:2H2O+2e-=H2↑+2OH-
B.试纸Ⅰ红色区长度大于蓝色区:说明单位时间内OH-的迁移速度快于H+
C.试纸Ⅱ中的现象说明此浓度下迁移的主要离子是Na+和SO42-
D.预测10min后,试纸Ⅰ红蓝区之间又会出现黄色区域
【答案】B
【解析】
电解硫酸钠溶液,在阳极a、c上是氢氧根离子放电,溶液显示酸性,试纸显示红色,在阴极b、d上是氢离子得电子的还原反应,溶液显示碱性,试纸显示蓝色,对比试纸I和试纸Ⅱ的现象分析解答。
A.b、d极均为阴极,H+在阴极上发生还原反应生成氢气,促进水的电离,溶液中OH-浓度增大,pH试纸变蓝,故A不选;
B.试纸I红色区和蓝色区不断向中间扩展,相遇时红色区约2.7cm,蓝色区约1.3cm,此现象说明此环境中H+的迁移速率比OH-快,故B错误;
C.试纸Ⅱ中c极上是氢氧根离子放电,溶液显示酸性,附近试纸变红,d极上是氢离子放电,溶液显示碱性,附近试纸变蓝,一段时间后,两极颜色范围扩大不明显,试纸大部分仍为黄色,此现象说明此浓度下迁移的主要离子不是H+、OH-,可能是Na+和SO42-,故C正确;
D.10min后,试纸Ⅰ中H+和OH-向两边继续迁移,氢离子和氢氧根离子反应生成水,溶液显中性,红蓝区之间又会出现黄色区域,故D正确;
故选B。

【题目】某蓄电池的反应为NiO2+Fe+2H2O![]() Fe(OH)2+Ni(OH)2。
Fe(OH)2+Ni(OH)2。
(1)该蓄电池放电时,发生还原反应的物质是________;
(2)为防止远洋轮船的钢铁船体在海水中发生电化学腐蚀,通常在船体上镶嵌Zn块,或与该蓄电池的_____ (填“正”或“负”)极相连。
(3)精炼铜时,粗铜应与直流电源的________(填“正”或“负”)极相连。精炼过程中,电解质溶液中的c(Fe2+)、c(Zn2+)会逐渐增大而影响进一步电解。甲同学设计如下除杂方案:
![]()
已知:
沉淀物 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
开始沉淀时的pH | 2.3 | 7.5 | 5.6 |
完全沉淀时的pH | 3.9 | 9.7 | 6.4 |
①实验操作I的名称________ 则加入H2O2的目的是____________________。
②乙同学认为应将方案中的pH调节到8,你认为此观点_____(填“正确”或“不正确”),理由是_________。
(4)该蓄电池充电时阴极的电极反应式为_________________。