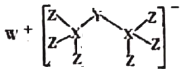
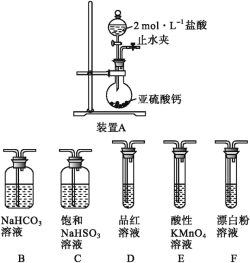
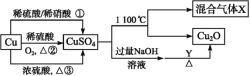
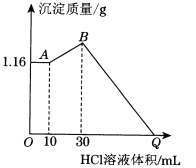
Ő‚ńŅńŕ»›
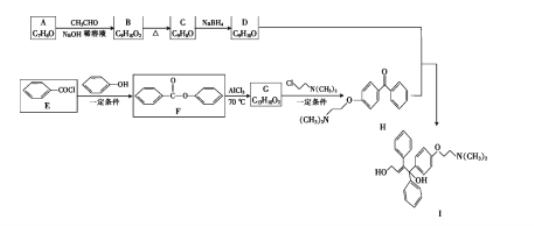
°ĺŐ‚ńŅ°Ņ”…∑ľŌ„ĽĮļŌőÔ A ļÕ E ő™‘≠ŃŌļŌ≥…ŤŘťŕňŠÕ–»ū√◊∑“Ķń÷–ľšŐŚ I Ķń“Ľ÷÷ļŌ≥…¬∑ŌŖ»ÁÕľňý ĺ:
“—÷™:ĘŔCH3CHO+CH3CHO ![]() CH3CH(OH)CH2CHO£ĽĘŕCH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO£ĽĘŕCH3CH(OH)CH2CHO ![]() CH3CH=CHCHO+H2O°£
CH3CH=CHCHO+H2O°£
ĽōīūŌ¬Ń–ő Ő‚:
£®1£©A ĶńĽĮ—ß√Ż≥∆ «__________£¨I ÷–ļ¨—űĻŔń‹ÕŇĶń√Ż≥∆ «_______________°£
£®2£©A …ķ≥… B Ķń∑ī”¶ņŗ–Õ «__________°£
£®3£©D ĶńĹŠĻĻľÚ Ĺő™__________°£
£®4£©E …ķ≥… F ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™__________°£
£®5£©∑ľŌ„ĽĮļŌőÔX «G ĶńÕ¨∑÷“žĻĻŐŚ,X ∑÷◊”÷–≥żĪĹĽ∑Õ‚≤Ľļ¨∆šňŻĽ∑◊īĹŠĻĻ,«“ĪĹĽ∑…Ō÷Ľ”–3 łŲ»°īķĽý,Xń‹”Ž NaHCO3 ∑ī”¶…ķ≥… CO2,∆šļňīŇĻ≤’Ů«‚∆◊”– 4 ◊ť∑Ś,∑Ś√śĽż÷ģĪ»ő™ 6°√2°√1°√1°£∑ŻļŌ…Ō Ų“™«ůĶńX ĶńĹŠĻĻľÚ Ĺő™__________(–ī“Ľ÷÷)°£
£®6£©–ī≥Ų”…¬»““ÕťļÕľ◊»©ő™‘≠ŃŌ÷∆ĪłĽĮļŌőÔ C(CH2OH)4 ĶńļŌ≥…¬∑ŌŖ__________°£(őřĽķ ‘ľŃ»ő”√)
°ĺīūįł°ŅĪĹľ◊»© Ű«Ľý°Ę√—ľŁ ľ”≥…∑ī”¶ ![]()
![]() +
+![]()
![]()
![]() +HCl
+HCl 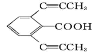 CH3CH2Cl
CH3CH2Cl![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]() (CH2OH)3CCHO
(CH2OH)3CCHO![]() C(CH2OH)4
C(CH2OH)4
°ĺĹ‚őŲ°Ņ
łýĺ›–ŇŌĘŅ…÷™£¨»©»©Ō»∑Ę…ķľ”≥…∑ī”¶°ĘļůŌŻ»•∑ī”¶∑ī”¶£¨…ķ≥…Ō©»©£Ľňý“‘ĪĹľ◊»©£®C7H6O£©ļÕ““»©‘ŕľÓ–‘Ľ∑ĺ≥Ō¬∑ī”¶…ķ≥…B(C9H10O2)£¨ĹŠĻĻľÚ Ĺő™£ļ 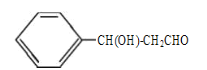 £¨Cő™
£¨Cő™ £Ľ‘ŕNaBH4ŐűľĢŌ¬£¨ĪĽĽĻ‘≠ő™D(C9H10O£©:
£Ľ‘ŕNaBH4ŐűľĢŌ¬£¨ĪĽĽĻ‘≠ő™D(C9H10O£©:![]() £Ľł√”–ĽķőÔ£®D£©”Ž
£Ľł√”–ĽķőÔ£®D£©”Ž ∑Ę…ķ∑ī”¶…ķ≥…
∑Ę…ķ∑ī”¶…ķ≥… £¨ĺ›“‘…Ō∑÷őŲĹÝ––Ĺ‚īū°£
£¨ĺ›“‘…Ō∑÷őŲĹÝ––Ĺ‚īū°£
(1)”–Ő‚ł…–ŇŌĘľįAĶńĽĮ—ß ĹŅ…÷™£¨AĶń√Ż≥∆ «ĪĹľ◊»©£¨Iļ¨”–ĶńĻŔń‹ÕŇ «Ű«Ľý£¨√—ľŁ£¨
Ļ īūįłő™£ļĪĹľ◊»© £ĽŰ«Ľý°Ę√—ľŁ
£®2£©A …ķ≥… BĶńĻż≥Ő «ŐľŐľň꾣ĪšĶ•ľŁ£¨ňý“‘∑ī”¶ņŗ–Õ «ľ”≥…∑ī”¶£¨
Ļ īūįłő™£ļľ”≥…∑ī”¶
£®3£©łýĺ›Ő‚“‚Ņ…“‘Õ∆≥ŲĶńĹŠĻĻľÚ Ĺ «![]() £¨
£¨
Ļ īūįł «£ļ![]() £Ľ
£Ľ
£®4£©EļÕĪĹľ◊īľ∑ī”¶…ķ≥…F£¨”–EļÕFĶńĹŠĻĻľÚ ĹŅ…÷™∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ «
![]() +
+![]()
![]()
![]() +HCl£¨
+HCl£¨
Ļ īūįłő™£ļ![]()
![]()
![]()
![]() +HCl£Ľ
+HCl£Ľ
£®5£©Xń‹”Ž NaHCO3 ∑ī”¶…ķ≥… CO2£¨ļ¨”–ĶńĻŔń‹ÕŇ «Ű»Ľý£¨ļňīŇĻ≤’Ů«‚∆◊”– 4 ◊ť∑Ś£¨ňĶ√ų”–ňń÷÷«‚‘≠◊”£¨∑Ś√śĽż÷ģĪ» «6°√2°√1°√1£¨ňĶ√ųňń÷÷«‚ňýļ¨«‚‘≠◊”ĶńłŲ ż∑÷Īū «6£¨2£¨1£¨1£¨Ņ…“‘Õ∆≥ŲĹŠĻĻľÚ Ĺ « £Ľ
£Ľ
Ļ īūįłő™£ļ
£®6£©”–Ő‚ł…–ŇŌĘ£¨Ņ…“‘Õ∆≥Ų”…¬»““ÕťļÕľ◊»©ő™‘≠ŃŌ÷∆ĪłĽĮļŌőÔ C(CH2OH)4 ĶńļŌ≥…¬∑ŌŖCH3CH2Cl![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]() (CH2OH)3CCHO
(CH2OH)3CCHO![]() C(CH2OH)4
C(CH2OH)4