��Ŀ����
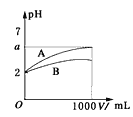
����Ŀ����ͼ��ʾ��ijͬѧ���һ������(CH3OCH3)ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ��

(1)д�������ĵ缫��Ӧʽ________________________������
(2)ʯī�缫(C)�ĵ缫��ӦʽΪ___________________��
(3)��Ӧһ��ʱ�����װ��������NaOH��Ҫ�� __________(��������������ʯī����)����
(4)�����ͭ�к���п���������ʣ���װ����п���ڽ���ͭ�ŵ������Һ����Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ�_________ (����������������С������������)
(5)���ڱ�״���£���2.24L�����μӷ�Ӧ��������װ�������缫�����ɵ������ڱ�״���µ����Ϊ__________________
���𰸡�CH3OCH3-12e-+16OH-=2CO32-+11H2O 2Cl-��2e-=Cl2�� ���� ��С 4.48L
��������
ȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�����ԭ��أ�Ͷ��ȼ�ϵĵ缫�Ǹ�����Ͷ���������ĵ缫���������׳�Ϊȼ�ϵ�أ��ڸõ���У�ͨ�����ĵ缫Ϊ������ͨ���ѵĵ缫Ϊ�������ҳ��У�Fe�缫Ϊ������C�缫Ϊ�����������У���ͭΪ��������ͭΪ������
(1)ͨ����ѵĵ缫Ϊ�������ڸõ缫������ʧ���ӣ����ת��ΪCO32-��H2O��
(2)ʯī�缫(C)Ϊ�������ڸõ缫��Cl-ʧ��������Cl2��
(3) ��װ���У�����H2O�ŵ磬����H2��OH-����������Na+ͨ�������ӽ���Ĥ���������������Է�Ӧһ��ʱ�������NaOH��Ҫ����������
(4)�����ͭ�к���п���������ʣ���װ����п���ڽ���ͭ�ŵ������Һ�������缫����ʽ�ֱ�ΪZn-2e-=Zn2+��Cu-2e-=Cu2+������ת�Ƶ��������֪���������ܽ��ͭС��������������ͭ�����Ա�װ���з�Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ���С��
(5)���ڱ�״���£���2.24L�����μӷ�Ӧ��������װ�������缫�����ɵ�H2����������ݵ����غ���м��㡣
(1)ͨ����ѵĵ缫Ϊ�������ڸõ缫������ʧ���ӣ����ת��ΪCO32-��H2O�������ĵ缫��ӦʽΪCH3OCH3-12e-+16OH-=2CO32-+11H2O����Ϊ��CH3OCH3-12e-+16OH-=2CO32-+11H2O��
(2)ʯī�缫(C)Ϊ�������ڸõ缫��Cl-ʧ��������Cl2���缫��ӦʽΪ2Cl-��2e-=Cl2������Ϊ��2Cl-��2e-=Cl2����
(3) ��װ���У�����H2O�ŵ磬����H2��OH-����������Na+ͨ�������ӽ���Ĥ���������������Է�Ӧһ��ʱ�������NaOH��Ҫ������������Ϊ��������
(4) �����ͭ�к���п���������ʣ���װ����п���ڽ���ͭ�ŵ������Һ�������缫����ʽ�ֱ�ΪZn-2e-=Zn2+��Cu-2e-=Cu2+������ת�Ƶ��������֪���������ܽ��ͭС��������������ͭ�����Ա�װ���з�Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ���С����Ϊ����С��
(5)���ڱ�״���£���2.24L�����μӷ�Ӧ��������װ�������缫�����ɵ�H2����������ݵ����غ㽨�����¹�ϵʽ��O2��4e-��2H2��V(H2)=2V(O2)=2��2.24L=4.48L����Ϊ��4.48L��

����Ŀ����������ʵ���������ó��Ľ�����ȷ����
ѡ�� | ʵ������ | ʵ����� |
A | ����Ƭ�ֱ�Ͷ��Ũ��ϡ�����У�ǰ�������������߷�Ӧ���� | ϡ����������Ա�Ũ�����ǿ |
B | ���з�̪��Na2CO3��Һ�м���BaCl2��Һ����ɫ��dz | Na2CO3��Һ�д���ˮ��ƽ�� |
C | ij��Һ�еμӹ�����ˮ������ɫ�����Ҳ��ܽ� | ����Һ��һ������Mg2+ |
D | ��ˮ��ͨ��SO2����ˮ��ɫ | SO2����Ư���� |
A. A B. B C. C D. D
����Ŀ��˫��ˮ(��������)������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ͬѧ��Χ���Ź������չ�˵����о���ʵ�顣
I.����
(1)�������ⳣ����������ɱ����Ư�ȷ������Ҫԭ����________��
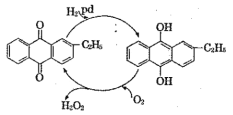
(2)�Ʊ���������Ŀǰ��õ����һ�������������Ҫ���̿�������ͼ��ʾ���˹������ܷ�ӦʽΪ________��
��.���ȶ���ʵ���о�
(3)Ϊ��̽���¶ȡ���������������� H2O2�ķֽ����ʵ�Ӱ�죬ij��ȤС��ͬѧ�������������ʵ�飬����ʵ�������Ѿ�����������С�
ʵ���� | T/X | H2O2��ʼŨ��/mol��L-1 | FeCl3��ʼŨ��/mol��L-1 |
I | 20 | 1.0 | _______ |
II | 50 | 1.0 | 0 |
III | 50 | ___________ | 0.1 |
ʵ��I�����о�______�Էֽ����ʵ�Ӱ�죬ʵ����о������Էֽ����ʵ�Ӱ�죬��ʵ�����H2O2��ʼŨ��ӦΪ________mol��L-1��
III.�������⺬����ʵ��ⶨ
��ȤС��ͬѧ��0.1000mol��L-1���Ը�����ر���Һ�ζ������й�������ĺ�������Ӧԭ��Ϊ2MnO4-+5H2O2+6H+=2Mn2+ +8H2O+5O2��
(4)����Һ����ȡ25.00mL����������ƿ�У��ظ��ζ��ĴΣ�ÿ�����ĵ�KMnO4����Һ������±���ʾ��
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
���(mL) | 17.10 | 18.10 | 18.00 | 17.90 |
���������й��������Ũ��Ϊ________mol��L-1(������λ��Ч���֣���