��Ŀ����
[14��]��֪��I2��2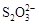

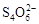 ��2I�D��������ʵ��ܶȻ��������±���
��2I�D��������ʵ��ܶȻ��������±���
| ���� | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
| Ksp | 2.2��10��20 | 2.6��10��39 | 1.7��10��7 | 1.3��10��12 |
���˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2?2H2O���塣
��2���ڿ�����ֱ�Ӽ���CuCl2?2H2O����ò���������ˮCuCl2��ԭ����_______________�����û�ѧ����ʽ��ʾ������CuCl2?2H2O����õ�������ˮCuCl2�ĺ���������_______��
��3��ijѧϰС���á���ӵ��������ⶨ����CuCl2?2H2O�������������������I�D������Ӧ�������������ʣ��Ĵ��ȣ��������£�ȡ0.36 g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫ��������0.1000 mol/L Na2S2O3����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ20.00 mL��
�ٿ�ѡ��___________���ζ�ָʾ�����ζ��յ��������_________________��
��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪ______________________________��
�۸�������CuCl2?2H2O�������ٷ���Ϊ___________________________��
(14��)
(1)Cu(OH)2��Cu2(OH)2CO3
2.6��10��9 mol��L-1
(2)2CuCl2��2H2O Cu(OH)2��CuCl2+2HCl+2H2O(��Ҫ����д��Cu(OH)2��Cu(OH)Cl��CuO����)
Cu(OH)2��CuCl2+2HCl+2H2O(��Ҫ����д��Cu(OH)2��Cu(OH)Cl��CuO����)
�ڸ����HCl�����м�����ˮ
(3)�ٵ�����Һ
��ɫ��ȥ������һ��ʱ���ɫ
��2Cu2++4I-=2CuI��+I2
��95��
��1��CuO ��2��CuCl2+H2O CuO+2HCl��CuCl2?2H2O������HCl�����м�����ˮ
CuO+2HCl��CuCl2?2H2O������HCl�����м�����ˮ
����

��ϰ��ϵ�д�
�����Ŀ
������, Ũ�Ⱦ�Ϊ0. 1 mol/L��6����Һ��pH���±�:
| ��� | a | b | c | d | e | f |
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | NaAlO2 |
| pH | 8. 8 | 9. 7 | 11. 6 | 10. 3 | 11. 1 | 11. 3 |
��2��NaAlO2��Һ�ʼ��Ե�ԭ���� (�����ӷ���ʽ��ʾ)������Һ�����������õ��Ĺ�������� ��
��3����ϱ������ݷ���, ��0. 1 mol/L��CH3COONa��Һ��ˮ�ĵ���̶���ͬ��������(����ĸ����)��
A.pH="8." 8��NaOH��Һ B. pH="5." 2��NH4Cl��Һ C.pH="5." 2������
D. 0. 1 mol/L��NaCN��Һ E.pH="8." 8��Na2CO3��Һ
��4����Ũ�Ⱦ�Ϊ0. 1 mol/L��b��c��������, ������Һ�и�����Ũ�ȹ�ϵ��ȷ���� ��
A.c(Na+)= c(CO32-) +c(HCO3-) +c(H2CO3)
B. 2c(Na+)=3c(CO32-) +3c(HCO3-) +3c(H2CO3)
C. c(OH-)= c(H+) +c(HCO3-) +2c(H2CO3)
D. c(Na+) +c(H+) = 2c(CO32-) +c(HCO3-) +c(OH-)
E. c(Na+)>c(HCO3-)> c(CO32-) > c(OH-)> c(H+)
F. c(Na+)> c(CO32-) > c(HCO3-) > c(H+)> c(OH-)
��5��0.2 mol/LHCl��0.1 mol/L NaAlO2��Һ����������Һ������Ũ��˳��Ϊ ��
��1��ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᡣ
�ٵζ�����ͼ��ʾ���� �ζ���ʢװ��Ũ�ȵ�����������Һ����ס����ҡ�����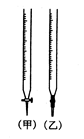
���õζ��ķ������ⶨ�����Ũ�ȣ�ʵ������������ʾ��
| ʵ���� | ����HCl��Һ�����(mL) | ����NaOH��Һ�����(mL) |
| 1 | 20.00 | 23.00 |
| 2 | 20.00 | 23.10 |
| 3 | 20.00 | 22.90 |
��δ֪�����Ũ��Ϊ��������λ��Ч���֣�_______________��
�����в�����ʹ����õ������Ũ��ƫ�͵���__________��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ���
��2��ij����С��Ϊ�˲ⶨij�Ȼ���(SrCl2)��Ʒ�Ĵ��ȣ���������·�������ȡ1.0 g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO3 2.38 g��AgNO3��Һ(��Һ�г�Cl���⣬����������Ag����Ӧ���ɳ���������)��Cl������ȫ��������Ȼ���ú�Fe3������Һ��ָʾ������0.2 mol��L��1��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag����AgSCN��ɫ��������ʽ�������Բⶨ�Ȼ�����Ʒ�Ĵ��ȡ�
��ش��������⣺
���жϵζ��ﵽ�յ��������_______________________________________________��
�ڿ���Ag����Fe3������������Һ�еĴ�����ʽ����ʵʩ�ζ�����Һ�Գ�_____(ѡ����ԡ��������ԡ����ԡ�)Ϊ�ˡ�
�����յ㵽��֮ǰ�ĵζ������У����ֳ����������������Ag�����費�Ͼ���ҡ����ƿ�������ʹn(Cl��)�IJⶨ���________(ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��

 2G��g��������
2G��g��������
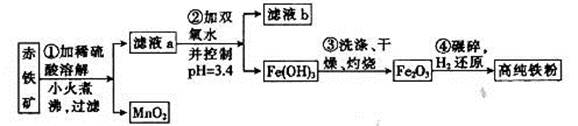
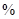 Fe2O3��3.6% FeO��������Al2O3��MnO2��CuO�ȡ�
Fe2O3��3.6% FeO��������Al2O3��MnO2��CuO�ȡ�
 Cu(OH) 2+2H+��ƽ�ⳣ��K=_______��
Cu(OH) 2+2H+��ƽ�ⳣ��K=_______��
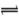 X2��+H+���ش��������⣺
X2��+H+���ش��������⣺ ��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��
��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��