��Ŀ����
����Ŀ�������Ǽס��ҡ�����λͬѧ��ȡ���������Ĺ��̣�������벢Э������������ʵ������
[ʵ��Ŀ��]��ȡ��������
[ʵ��ԭ��]�ס��ҡ�����λͬѧ����ȡ�Ҵ���������Ũ�����Ϲ��ȵķ�����ȡ����������
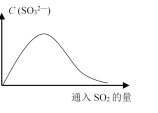
[װ�����]�ס��ҡ�����λͬѧ�ֱ��������������ʵ��װ�ã�
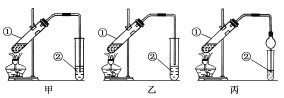
��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã��Ϻ�������________(ѡ����������������)����ͬѧ����װ���еIJ����ܸij������θ���ܣ��������������⣬��һ��Ҫ������____________________��
[ʵ�鲽��]
��.����ͬѧѡ���װ����װ���������Թ����ȼ���3 mL�Ҵ�������ҡ���»�������2 mLŨ������ҡ�ȣ���ȴ���ټ���2 mL�����
��.���Թ̶ܹ�������̨�ϣ�
��.���Թܢ��м��������ı���Na2CO3��Һ��
��.�þƾ��ƶ��Թܢټ��ȣ�
��.���۲쵽�Թܢ�������������ʱֹͣʵ�顣
[��������]
(1)�����װ��ʵ��װ�ã�������Ʒǰ��Ӧ���_____________
(2)д���Թܢ��з�����Ӧ�Ļ�ѧ����ʽ��____________(ע����Ӧ����)��
(3)�Թܢ��б���Na2CO3��Һ��������_____________
(4)���Թܢ��з��������������ʵ�������____________
���𰸡��� ��ֹ���� װ�õ������� CH3COOH��C2H5OH��CH3COOC2H5��H2O �����Ҵ�����ȥ������������������ܽ�ȣ�ʹ��ֲ����� ��Һ
��������
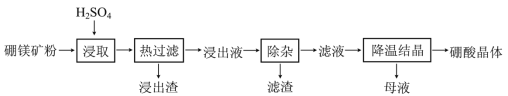
����ʵ������ȡ����������ʵ��ԭ����ע�������������⡣
�����Ҵ�������������������ӷ���ʵ������ȡ��������ʱ�������Ҵ����������ʣ��Ҵ�������ˮ����������̼������Һ��Ӧ�����ײ������������ݼ�װ���ǵ��ܲ���Һ���£����ײ�����������װ���ǵ���û�в���Һ���£������ײ�������������ѡ����װ�����������������ȡ����װ��ͨ������©������������ɷ�ֹ�������ʴ�Ϊ���ң���ֹ������
(1) �����Ҵ�������������������ӷ������Բ����װ��ʵ��װ�ã�������Ʒǰ��Ӧ���װ�õ������ԣ��ʴ𰸣�װ�õ������ԣ�
(2) �Թܢ�����������Ҵ���Ũ����������·���������Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH![]() CH3COOC2H5+H2O ���ʴ�Ϊ��CH3COOH
CH3COOC2H5+H2O ���ʴ�Ϊ��CH3COOH![]() C2H5OH
C2H5OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
(3) �����Ҵ���������ӷ��������ȡ�����������л�������������ʣ����ñ���Na2CO3��Һ��ȥ�Ҵ������ᣬͬʱ���������ڱ���Na2CO3��Һ�е��ܽ�Ƚ�С�����Դ�Ϊ�������Ҵ�����ȥ������������������ܽ�ȣ�ʹ��ֲ�������
(4) ��Ϊ����������״Һ�壬�����ڱ���Na2CO3��Һ�������ܶȱ�ˮС�����Ը�����Һ�Ϸ������÷�Һ©�����з�Һ���ɴ��Թܢ��з���������������𰸣���Һ��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�����Ŀ��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
������ | CO |
������ | Al3����Fe3����Mg2����NH4+��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y���(V)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
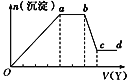
A.��Y�����ᣬ��Oa��ת��Ϊ����������(�ϱ��У���ͬ)ֻ��[Al(OH)4]��
B.��Y�����ᣬ����Һ�п��ܺ��е���������Al3��
C.��Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪAl(OH)3��OH��=[Al(OH)4]��
D.��Y��NaOH��Һ����X��Һ��ֻ�����������ӣ���Al3����Fe3����NH4+��Cl��