��Ŀ����
1���ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ��ͼ�����������գ�
��1�����ƺ�õ��ij����ɷ���CaCO3��Mg��OH��2
��2�������к�����Ca2+��Mg2+��SO42-��Ϊ��Ч��ȥ��Щ���ӣ������Լ��ĺ���˳��Ϊbc
a��NaOH��Na2CO3�����Լ�������
b��NaOH�����Լ���Na2CO3������
c�����Լ���NaOH��Na2CO3������
��3��д�����ʱ�����Ļ�ѧ����ʽ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2��
��4�����ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�������ᾧ����ȴ�����ȹ��ˣ���д�������ƣ���ȥNaCl��
���� �����̿�֪�����μ�ˮ�����ܽ��NaOH��ȥþ���ӣ���̼���Ƴ�ȥ�����ӣ����ƺ�õ��ij���ΪCaCO3��Mg��OH��2�����ƺ��ʳ��ˮ�������NaOH��������������������Σ�NaCl�ȣ��õ���Ũ�ȵ�NaOH��Һ���Դ������
��� �⣺��1��Ca2+��Mg2+��������̼���ơ�NaOH��Ӧת��Ϊ���������ӷ�Ӧ�ֱ�ΪCa2++CO32-�TCaCO3����Mg2++2OH-�TMg��OH��2�������ƺ�õ��ij���ΪCaCO3��Mg��OH��2���ʴ�Ϊ��CaCO3��Mg��OH��2��
��2�������к�����Ca2+��Mg2+��SO42-��Ϊ��Ч��ȥ��Щ���ӣ�ѡNaOH��ȥþ���ӣ�̼���Ƴ�ȥ�����ӡ��Ȼ�����ȥ��������ӣ���̼����һ�����Ȼ���֮�ɳ�ȥ�����ı����ӣ�bc�����ϣ��ʴ�Ϊ��bc��
��3�����ʱ�����Ļ�ѧ����ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
��4��NaOH���ܽ�����¶ȵ����߶�����NaCl���ܽ�����¶ȵ�Ӱ�첻�������¶ȸߵ�ʱ�������������ƾ��弴�ɳ�ȥ�Ȼ��ƣ������������ƺ��Ȼ��Ʒ���ķ����������ᾧ����ȴ�����ȹ��ˣ��ʴ�Ϊ�������ᾧ�����ȹ��ˣ�
���� ���⿼����������ᴿ���ۺ�Ӧ�ü���ˮ��Դ���õȣ�Ϊ��Ƶ���㣬���շ��������еķ�Ӧ���������뷽��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д���Na2SO4����AlCl3����Al��OH��3����Al2O3����NaHCO3���ޣ�NH4��2CO3��
| A�� | �٢ڢۢܢݢ� | B�� | �٢ڢܢݢ� | C�� | �ڢܢݢ� | D�� | �ۢܢݢ� |
| A�� | �ǽ�����X��Y��Z | |
| B�� | ��̬�⻯������ȶ���X��Y��Z˳����� | |
| C�� | X��Y��Z������������Ӧˮ��������������ǿ | |
| D�� | X��Y��Z��������������� |
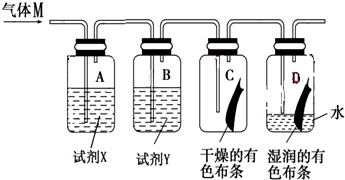
��1��װ��A��B�е��Լ�X���Լ�Y�ֱ���b������ĸ���ţ���
a��NaOH��Һ Ũ���� b��NaCl��Һ Ũ���� c��Ũ���� ʯ��ˮ
��2����C����ɫ��������ɫ��D����ɫ������ɫ�������Ư���Ե������Ǵ����ᣨ���������ƣ�����ʵ��֤����������ˮ��Ӧ����Ӧ�����ӷ���ʽΪCl+H2O=H++Cl-+HClO��
��3������ɫ��ѧ�ĽǶȷ���������ʵ��װ��ͼ�д���һ��ȱ�ݣ������ķ����Ǽ�һ��β������װ�ã���β������װ���ڷ�����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
��4��ʵ����Ϻ�D�е�ˮ��Һ���ʻ���ɫ��ȡ�����ֱ������֧�Թ��У���������ʵ��
| ʵ���� | �μӵ��Լ� | ʵ������ | ֤�����ڵ��� ���������ţ� |
| A | AgNO3 ��Һ | a��������ɫ���� | b��Cl- |
| B | ��ɫʯ����Һ | c���ȱ��ɫ����ɫ | d��H+��HClO |
| A�� | 100 mL 0.1 mol•L-1 Na2SO4��Һ�У�����������0.03NA | |
| B�� | ���³�ѹ�£�32 g O2-���������ӵ���ĿΪ17NA | |
| C�� | 1 mol Al3+��ȫˮ���������������������ӵ���ĿΪNA | |
| D�� | ��״���£�������ΪNA��N2��C2H4��������������ȷ�� |
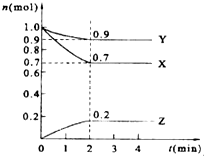 ij�¶�ʱ����2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ��
ij�¶�ʱ����2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��ı仯������ͼ��ʾ��