��Ŀ����
10�����������ṩ���������Լ��������֤SO2�������������л�ԭ�Ե�ʵ�飮��֪����SO2+Br2+2H2O��2HBr+H2SO4����FeS+2HCl��FeCl2+H2S����FeS����������ˮ������������������ˮ����ѡ�õ�������ͼ��ʾ����ѡ�õ��Լ��������ᣬ����ˮ����Na2SO3���壬��FeS���壬��Ʒ����Һ��������������Һ����Ũ���ᣮ�Իش��������⣺
��1��װ��A����˫�������������ܼ���Һ©����Բ����ƿ��װ�����ģ�
��2����ȡSO2����ѡ��װ��A�� ��A--D��ѡȡ������ĸ����ѡ�õ��Լ��Ǣۺ͢ߣ��Ӣ�--����ѡȡ�������֣���
��3����ȡH2S����ѡ��װ��B�� ��A--D��ѡȡ������ĸ����ѡ�õ��Լ��Ǣٺ͢ܣ��Ӣ�--����ѡȡ�������֣���
��4������SO2�����Ե�ʵ�������������е�Dװ�ã���A--D��ѡȡ������ĸ����ʵ��������ܹ۲쵽��ʵ��������װ���ڱ����е���ɫ�Ĺ���������Һ�����ɣ�����SO2��ԭ�Ե�ʵ�������������е�Cװ�ã�ʵ��������ܹ۲쵽��ʵ����������ˮ��ɫ��
��5��Ϊ�˷�ֹ��Ⱦ��������װ��D�ij�����Ӧͨ��Ӧ����ʢ��NaOH��Һ��Cװ��3�ڣ�
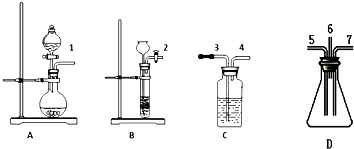
���� ��֤SO2�������������л�ԭ�Ե�ʵ�飬��ͼ��֪��A�з����ۢ����ʵķ�Ӧ����SO2��B�з����٢����ʵķ�Ӧ����H2S����C�м���ˮ��1��3��������ˮ��ɫ������SO2+Br2+2H2O=H2SO4+2HBr��4��5������ͬʱ2��7�������������������������D�з���2H2S+SO2=3S��+2H2O��Ϊ��ֹ��Ⱦ������Ȼ�������6��3��������ʱC��ΪNaOH��Һ����β���������Դ������
��� �⣺��֤SO2�������������л�ԭ�Ե�ʵ�飬��ͼ��֪��A�з����ۢ����ʵķ�Ӧ����SO2��B�з����٢����ʵķ�Ӧ����H2S����C�м���ˮ��1��3��������ˮ��ɫ������SO2+Br2+2H2O=H2SO4+2HBr��4��5������ͬʱ2��7�������������������������D�з���2H2S+SO2=3S��+2H2O��Ȼ�������6��3��������ʱC��ΪNaOH��Һ����β��������
��1��װ��A����˫�������������ܼ���Һ©����Բ����ƿ��װ�����ģ��ʴ�Ϊ����Һ©����Բ����ƿ��
��2����ȡSO2����ѡ��װ��A��Na2SO3+H2SO4=Na2SO4+H2O+SO2����������������֪��A���Լ�Ϊ�ۢߣ�
�ʴ�Ϊ��A���ۢߣ�
��3����ȡH2S����ѡ��װ��ΪB�����������ӷ���ʽ�ֱ�ΪFeS+2H+=H2S��+Fe2+��B���Լ�Ϊ�٢ܣ�
�ʴ�Ϊ��B���٢ܣ�
��4��D�з���2H2S+SO2=3S��+2H2O��SԪ�صĻ��ϼ۽��ͣ����ֶ�������������ԣ��۲쵽װ���ڱ����е���ɫ�Ĺ���������Һ�����ɣ�C�з���SO2+Br2+2H2O=H2SO4+2HBr��SԪ�صĻ��ϼ����ߣ����ֶ�������Ļ�ԭ�ԣ��۲쵽��ˮ��ɫ��
�ʴ�Ϊ��D��װ���ڱ����е���ɫ�Ĺ���������Һ�����ɣ�C����ˮ��ɫ��
��5����ʵ���ж����������⡢��������Ϊ�ж����壬������Ⱦ���������뾻�����ٷſգ���װ��D�ij�����Ӧ����ʢ��NaOH��Һ��Cװ��3�ڣ�
�ʴ�Ϊ��װ��D�ij�����Ӧ����ʢ��NaOH��Һ��Cװ��3�ڣ�
���� ���⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬���ջ�ԭ���������Ե�ʵ���з����Ļ�ѧ��Ӧ����ʵ�����Ϊ���Ĺؼ���ע��������ԭ��Ӧ��ʵ�鼼�ܡ���ѧ�뻷�����ۺϿ��飬��Ŀ�Ѷ��еȣ�

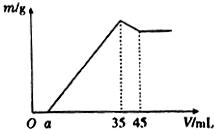 ȷ��ȡ6g��������Ʒ����Al2O3��Fe2O3��SiO2������ʢ��100mLijŨ��������Һ���ձ��У���ַ�Ӧ����ˣ�����Һ�м���10mol•L-l��NaoH��Һ�������ij�������m������NaOH��Һ�����V�Ĺ�ϵ��ͼ��ʾ��������������Һ�����ʵ���Ũ��Ϊ��������
ȷ��ȡ6g��������Ʒ����Al2O3��Fe2O3��SiO2������ʢ��100mLijŨ��������Һ���ձ��У���ַ�Ӧ����ˣ�����Һ�м���10mol•L-l��NaoH��Һ�������ij�������m������NaOH��Һ�����V�Ĺ�ϵ��ͼ��ʾ��������������Һ�����ʵ���Ũ��Ϊ��������| A�� | 3.50mol/L | B�� | 1.75mol/L | C�� | 0.85mol/L | D�� | ������ |
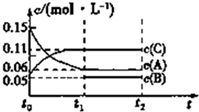 ��ij�ܱ������м���0.3mol A��0.1mol C��һ������B�������壮һ�������·�����Ӧ�������ʵ�Ũ����ʱ��仯���ͼ��ʾ[t0��t1�ε�c��B���仯δ����]��t1ʱ�̴ﵽƽ�⣬��Ӧ�����л�������ƽ����Է�������ʼ�ղ��䣮����˵����ȷ���ǣ�������
��ij�ܱ������м���0.3mol A��0.1mol C��һ������B�������壮һ�������·�����Ӧ�������ʵ�Ũ����ʱ��仯���ͼ��ʾ[t0��t1�ε�c��B���仯δ����]��t1ʱ�̴ﵽƽ�⣬��Ӧ�����л�������ƽ����Է�������ʼ�ղ��䣮����˵����ȷ���ǣ�������| A�� | �����������Ϊ2L | |
| B�� | ��ʼʱB�����ʵ���Ϊ0.08mol | |
| C�� | �÷�Ӧ�ķ���ʽΪ3A=2C+B | |
| D�� | ��t1=15 s������B��Ũ�ȱ仯��ʾ��t0��t1�ε�ƽ����Ӧ����Ϊ0.002 mol/��L•s�� |
| A�� | 0.3 mol•L-1 | B�� | 0.4 mol•L-1 | C�� | 0.45 mol•L-1 | D�� | 0.5 mol•L-1 |
