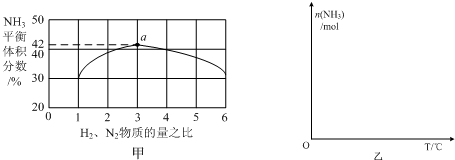
��Ŀ����
15��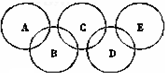 ��ͼ��ʾ��Ԫ������A��B��C��D��E���ֻ����ԲȦ���沿��ָ���ֻ����ﺬ��һ����ͬԪ�أ����ֻ����������ֶ�����Ԫ���γɣ�ÿ�ֻ��������������Ԫ�أ�A�ǹ�ҵ��ȡ�������Ҫԭ��֮һ��B��E������������������Ϊ18��B���ȶ������н�ǿ�������ԣ�E��������ԭ�ӹ��ɵķ��ӣ��������ȼ�ϣ�C�ǹ�ҵ�ƹ��ά����Ҫԭ�ϣ�D������������Ԫ�ص�ԭ�Ӹ���֮��Ϊ3��4������������Ϣ�ش��������⣺
��ͼ��ʾ��Ԫ������A��B��C��D��E���ֻ����ԲȦ���沿��ָ���ֻ����ﺬ��һ����ͬԪ�أ����ֻ����������ֶ�����Ԫ���γɣ�ÿ�ֻ��������������Ԫ�أ�A�ǹ�ҵ��ȡ�������Ҫԭ��֮һ��B��E������������������Ϊ18��B���ȶ������н�ǿ�������ԣ�E��������ԭ�ӹ��ɵķ��ӣ��������ȼ�ϣ�C�ǹ�ҵ�ƹ��ά����Ҫԭ�ϣ�D������������Ԫ�ص�ԭ�Ӹ���֮��Ϊ3��4������������Ϣ�ش��������⣺��1��A���ڹ�ҵ��ȡ������������Ӧ�Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��2��D�Ļ�ѧʽ��Si3N4��
��3��B�ĽṹʽΪH-O-O-H��
��4��B��E��Ӧ�����ɵ���G��һ�ֳ�����Һ��H���䷴Ӧ�Ļ�ѧ����ʽΪ2H2O2+N2H4=N2��+4H2O��
��5���û�ѧ����ʽ��ʾC����һ����;2C+SiO2$\frac{\underline{\;����\;}}{\;}$2CO��+Si��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$CO2��+Na2SiO3��
���� A�ǹ�ҵ��ȡ�������Ҫԭ��֮һ��ΪNH3��B����������������Ϊ18��B���ȶ������н�ǿ�������ԣ�������Ԫ�أ���֪BΪH2O2��C�к���OԪ�أ�C�ǹ�ҵ�ƹ��ά����Ҫԭ�ϣ�CΪSiO2��E����������������Ϊ18��E��������ԭ�ӹ��ɵķ��ӣ�ԭ��ƽ��������Ϊ3����E�к���HԪ�أ�EΪN2H4��D�к���SiԪ�أ�������HԪ�ػ�NԪ�أ���D������������Ԫ�ص�ԭ�Ӹ���֮��Ϊ3��4����DΪSi3N4���Դ˽����⣮
��� �⣺A�ǹ�ҵ��ȡ�������Ҫԭ��֮һ��ΪNH3��B����������������Ϊ18��B���ȶ������н�ǿ�������ԣ�������Ԫ�أ���֪BΪH2O2��C�к���OԪ�أ�C�ǹ�ҵ�ƹ��ά����Ҫԭ�ϣ�CΪSiO2��E����������������Ϊ18��E��������ԭ�ӹ��ɵķ��ӣ�ԭ��ƽ��������Ϊ3����E�к���HԪ�أ�EΪN2H4��D�к���SiԪ�أ�������HԪ�ػ�NԪ�أ���D������������Ԫ�ص�ԭ�Ӹ���֮��Ϊ3��4����DΪSi3N4��
��1��AΪΪNH3�������ڹ�ҵ��ȡ���ᣬ��Ӧ�ķ���ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��2�������Ϸ�����֪DΪSi3N4���ʴ�Ϊ��Si3N4��
��3��BΪH2O2������O-O�����ṹʽΪH-O-O-H���ʴ�Ϊ��H-O-O-H��
��4��H2O2��N2H4��Ӧ�����ɵ���G��һ�ֳ�����Һ��H��HΪˮ�����嵥��Ϊ�������䷴Ӧ�Ļ�ѧ����ʽΪ2H2O2+N2H4=N2��+4H2O��
�ʴ�Ϊ��2H2O2+N2H4=N2��+4H2O��
��5��CΪSiO2���������Ʊ����ʹ�����첣������Ӧ�ķ���ʽΪ2C+SiO2$\frac{\underline{\;����\;}}{\;}$2CO��+Si��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$CO2��+Na2SiO3��
�ʴ�Ϊ��2C+SiO2$\frac{\underline{\;����\;}}{\;}$2CO��+Si��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$CO2��+Na2SiO3��
���� ���⿼�����ʵ��ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�ѶȽϴ����ճ���18�����������ƶϵĹؼ�����Ҫѧ���Գ������ʵĽṹ�������������գ�

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д������Ӽ�����������۹��ۼ����ܼ��Լ����ݷǼ��Լ���
| A�� | �٢� | B�� | �٢ڢ� | C�� | �ۢ� | D�� | �٢ۢ� |
| A�� | ��ij��Ӧ�У�����Ӧ����е�������������������е�������ʱ���÷�Ӧ���� | |
| B�� | ͬ��ͬѹ�£�4Al��s��+3O2��g���T2Al2O3��s���ڳ��º͵�ȼ�����µġ�H��ͬ | |
| C�� | ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l������H=-53.7KJ/mol��������0.5molH2SO4��Ũ��Һ�뺬1molNaOH����Һ��ϣ��ų�����������53.7kJ | |
| D�� | ��Ҫ���ȵĻ�ѧ��Ӧһ�������ȷ�Ӧ |
 �ס��ҡ���������������ѧ������������мס��Ҿ�Ϊ���ʣ����ǵ�ת����ϵ��ͼ��ʾ��ijЩ�����Ͳ��ֲ�������ȥ��������˵������ȷ���ǣ�������
�ס��ҡ���������������ѧ������������мס��Ҿ�Ϊ���ʣ����ǵ�ת����ϵ��ͼ��ʾ��ijЩ�����Ͳ��ֲ�������ȥ��������˵������ȷ���ǣ�������| A�� | ����Ϊ��������ԭ�Ӱ뾶��������Ԫ�صĵ��ʣ�����Ϊ����ֻ��ΪNa2O2 | |
| B�� | �����������ᷴӦ������NaOH��Һ��Ӧ����������������������� | |
| C�� | ����������ϲ������̣��ұ�Ϊ18���ӷ��ӣ���Ϊ10���ӷ��ӣ����ҵ�ˮ��Һ���ܾ���Ư������ | |
| D�� | ���ס������캬��ͬһ��Ԫ�أ������������У���Ԫ�صĻ��ϼ��ɵ͵��ߵ�˳�����Ϊ���ף������� |
| A�� | 1.5 mol | B�� | 1 mol | C�� | 0.5 mol | D�� | 0 |
| A�� | 16 | B�� | 18 | C�� | 20 | D�� | 22 |
��H+�����ʵ���Ũ��
��OH-�����ʵ���Ũ��
��c��CH3COO-��/c��CH3COOH��
��c��H+��•c��OH-��
| A�� | �٢� | B�� | �� | C�� | �ڢ� | D�� | �ڢۢ� |
| �� ���� | IA | ��A | ��A | ��A | VA | ��A | VIIA | 0�� |
| 1 | �� | |||||||
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� |
 ��
����2������ЩԪ���У��۵���̬�⻯��������̬�⻯�ﷴӦ������Ϊð���̣�����ľ�������Ϊ���Ӿ��壮
��3������ЩԪ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ĵ���ʽΪ
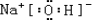 ��
����4���ĵ�����ڡ����γɵ�ij�ֻ����ﷴӦ�Ļ�ѧ����ʽΪ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��5���ڢߡ�������Ԫ���У��ǽ����Խ�ǿ����Cl�������Ԫ�ط��ţ�����֤����һ���۵Ļ�ѧ����ʽΪCl2+H2S=2HCl+S��
��6����ѧ�ҽ���Ԫ�����ڱ��о��ϳ����ض����ʵ������ʣ����ڽ�����ǽ������紦Ѱ�Ұ뵼����ϣ�
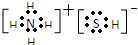 ��д��������Ӧ�Ļ�ѧ����ʽ��2NH4HS+O2=2S��+2NH3•H2O��NH3�ķе����H2S������ΪNH3����֮�������һ�ֱȷ��Ӽ���������ǿ����������
��д��������Ӧ�Ļ�ѧ����ʽ��2NH4HS+O2=2S��+2NH3•H2O��NH3�ķе����H2S������ΪNH3����֮�������һ�ֱȷ��Ӽ���������ǿ����������