��Ŀ����
1��ȡ3.86g�Ѵ���˽ϳ�ʱ���Na2SO3��ĩ�����ܺ�����������Na2SO4���������м���ijŨ�ȵ�����20ml������ʹ����������ȫ���ݳ�������Ũ��������ⶨ�������ڱ�״�������Ϊ560ml��Ȼ������Һ������0.5mol/L����������Һ50ml����Һ�в����˳�������ʱ��Һ��pH=7������1��ԭNa2SO3�Ĵ���
��2��������������ʵ���Ũ��
��3����pH=7����Һ�У�������Ҫ�ٵμ�Ba��OH��2��Һ���ٺ���ʱ�������ij����������ӣ�
���� ��1����������õ�560mL����ΪSO2������Sԭ���غ㣬��n��Na2SO3��=n��SO2�����ٸ���m=nM����m��Na2SO3�����������㴿�ȣ�
��2����ϣ�1���п��Լ�����Ʒ��n��Na2SO4��������������������Һ�����ԣ���ǡ�÷�Ӧ�õ�Na2SO4��BaSO4�����ݱ������غ��֪n��BaSO4��=n[Ba��OH��2]���ٸ����������غ���㷴Ӧ����Һ�е�n��Na2SO4������������������غ����n��H2SO4�����ٸ���c=$\frac{n}{V}$����c��H2SO4����
��3���ټ����Ba��OH��2����Һ��Na2SO4��Ӧ����n[Ba��OH��2]=n��Na2SO4��������������ҪBa��OH��2��Һ�����
��� �⣺��1���������ᷢ����Ӧ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O���õ�560mL����ΪSO2����n��Na2SO3��=n��SO2��=$\frac{0.56L}{22.4L/mol}$=0.025mol��m��Na2SO3��=0.025mol��126g/mol=3.15g��
ԭNa2SO3�Ĵ���Ϊ$\frac{3.15g}{3.86g}$��100%=81.6%��
��ԭNa2SO3�Ĵ���Ϊ81.6%��
��2����Ʒ��m��Na2SO4��=3.86g-3.15g=0.71g������Ʒ��n��Na2SO4��=$\frac{0.71g}{142g/mol}$=0.005mol������������������Һ�����ԣ���ǡ�÷�Ӧ�õ�Na2SO4��BaSO4�����ݱ������غ��֪n��BaSO4��=n[Ba��OH��2]=0.05L��0.5mol/L=0.025mol�������������غ㣬��Ӧ����Һ�е�n��Na2SO4��=n��Ʒ��Na2SO4��+n��Na2SO3��=0.005mol+0.025mol=0.03mol��������������غ㣺n��H2SO4��=n��BaSO4��+n��Һ��Na2SO4��-n��Ʒ��Na2SO4��=0.025mol+0.03mol-0.005mol=0.05mol������c��H2SO4��=$\frac{0.05mol}{0.02L}$=2.5mol/L��
�������Ũ��Ϊ2.5mol/L��
��3���ټ����Ba��OH��2����Һ��Na2SO4��Ӧ����n[Ba��OH��2]=n��Һ��Na2SO4��=0.03mol������ҪBa��OH��2��Һ���Ϊ$\frac{0.03mol}{0.5mol/L}$=0.06L����Ϊ60mL��
����Ҫ����������Һ�������Ϊ60mL��
���� ���⿼��������йؼ��㣬��Ŀ�����漰��Ӧ�϶࣬ע�������غ�����𣬲��ؿ���ѧ�����������������Ѷ��еȣ�

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�| A�� | ������ˮ | B�� | CH2C12ֻ��һ�ֽṹ | ||
| C�� | ��ȼ���� | D�� | ��ʹ�������������Һ��ɫ |
| a | |||||||||||||||||
| f | h | i | |||||||||||||||
| b | e | j | |||||||||||||||
| c | d | g | k | ||||||||||||||
| l | |||||||||||||||||
��2����д��j�ĵ�����a��h�γɵĻ��������Ӧ�Ļ�ѧ����ʽCl2+H2O=HCl+HClO��
��3����Ƚ�b��e��j����Ԫ�صĵ縺���ɴ�С��˳��Cl��Al��Mg��дԪ�ط��ţ���������Ԫ�صĵ�һ�������ɴ�С��˳��Cl��Mg��Al��дԪ�ط��ţ���
��4��g��e����Ԫ�ص�����������Ӧ��ˮ����Ļ�ѧ�������ƣ���д��eԪ������������Ӧ��ˮ������a��c��h����Ԫ���γɵĻ����ﷴӦ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
��5��j��k��l����Ԫ��֮������ԭ�Ӹ�����1��1�����γɻ������Щ�������������������ЩԪ�ص��ʵ����ʣ���д��k��l�Ļ�����ĵ���ʽ
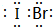 �������ɦļ�������ԭ�ӹ�����ص��̶���д���γɵĹ��ۻ��������ӡ����ۡ�����
�������ɦļ�������ԭ�ӹ�����ص��̶���д���γɵĹ��ۻ��������ӡ����ۡ����� | A�� | ʹ�øߴ��ȵ�п�� | B�� | �μӼ�������ͭ��Һ | ||
| C�� | ʹ��ŨH2SO4 | D�� | ʹ��ŨHNO3 |
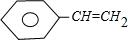 +Br2��
+Br2�� ����Ӧ����Ϊ�ӳɷ�Ӧ��
����Ӧ����Ϊ�ӳɷ�Ӧ�� ��
��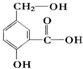 ������1mol�ĸ��л���������Ľ����Ʒ�Ӧ���Բ���1.5molH2��1mol���л�����Ը�1molNaHCO3��Ӧ��1mol���л�����Ը�2molNaOH��Ӧ��
������1mol�ĸ��л���������Ľ����Ʒ�Ӧ���Բ���1.5molH2��1mol���л�����Ը�1molNaHCO3��Ӧ��1mol���л�����Ը�2molNaOH��Ӧ��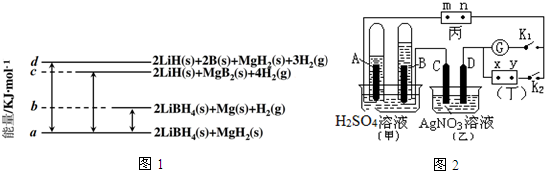
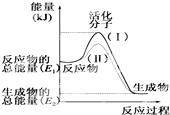 ������۲���ͼ��Ȼ��ش����⣺
������۲���ͼ��Ȼ��ش����⣺