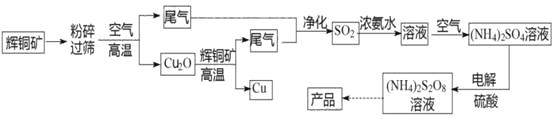
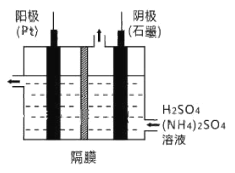
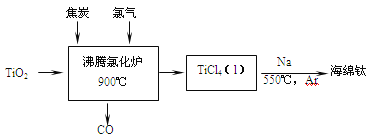
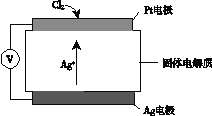
��Ŀ����
����Ŀ�������й�˵���в���ȷ����( )
A.ij�¶�ʱ�Ļ����Һ��c(H+) =![]() mol��L-1��˵������Һ������(KwΪ���¶�ʱˮ�����ӻ�����)
mol��L-1��˵������Һ������(KwΪ���¶�ʱˮ�����ӻ�����)
B.�����£���ˮ�������c(H+)=10-12mol��L-1����Һ��pH����Ϊ2��12
C.�����£�pH=3��CH3COOH��pH=11��NaOH��Һ�������Ϻ����Һ�У�c(H+)��c(OH-)
D.������pH=7��CH3COOH��CH3COONa�����Һ�У�c(Na+)=c(CH3COO-)+c(CH3COOH)
���𰸡�D
��������
A��ij�¶�ʱ�Ļ����Һ��c(H+) =![]() mol��L-1����Kw=c��H������c��OH������˵��c��H����=c��OH����������Һһ��Ϊ���ԣ���A��ȷ��
mol��L-1����Kw=c��H������c��OH������˵��c��H����=c��OH����������Һһ��Ϊ���ԣ���A��ȷ��
B�������£�Kw=c��OH������c��H����=10-14����ˮ��c��H����=c��OH����=1.0��10-7mol��L��1��������ij��Һ�У���ˮ�������c��H����ˮ=1.0��10-12mol��L��1��10-7mol��L��1��˵������Һ�е���������ˮ�ĵ��룬�����ܵ���������ӻ����������Ӿ�������ˮ���룬������ʿ����������ǿ�����ʽ�Σ����Ϊ����Һ����pH=2�����Ϊ����Һ����pH=12����B��ȷ��
C��CH3COOH�����ᣬ�ѵ��룬pH=3��CH3COOH��Һ�����Ũ�ȴ����£�pH=3��CH3COOH��pH=11��NaOH��Һ�������Ϻ����Һ�У������������Һ�����ԣ���c(H+)��c(OH-)����C��ȷ��
D��������pH=7��CH3COOH��CH3COONa�����Һ�����ԣ���c��H����=c��OH��������ϵ���غ��֪��c��Na����=c��CH3COO��������D����
��ѡD��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�