��Ŀ����
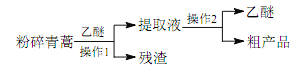
����Ŀ��ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ����ʣ�ʵ��װ������ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���
���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
�е㣨�棩 | -252��8 | -146 | -103��7 | -47��4 | 80��10 | 110��63 | 4827 |

��1�����Թ������ղ�������_____________�����ж�����;��������ת���Ϳ���ȡ����Ȳ��д����Ӧ�ڵĻ�ѧ����ʽ__________________________ ��
![]()
��2�������Թ��ռ��������ֲ�Ʒ�У���һ����ʹ���Ը��������Һ��ɫ�����ʣ�������Ϊ__________��
��3��������ƿ�۲쵽������_____________________________________����Ӧ�Ļ�ѧ����ʽ��__________________________��_________________________��
��4����������Ȼ�̼��Һ������գ�����ռ�����������______________��
���𰸡�C��̼ CaC2 +2H2O �� Ca (OH)2 + C2H2�� �ױ� ��Һ��ɫ CH2=CH2+Br2��CH2BrCH2Br CH3CH=CH2��Br2��CH3CHBrCH2Br ���顢����
��������
(1). �ɱ��и�����ķе����ݿ�֪���۱�ϩ�����ϼ�ǿ��ʱ���Թ��еIJ�����ΪC����ʯ����Ҫ�ɷ���̼���ƣ�̼������ˮ��Ӧ�����������ƺ���Ȳ��
(2). �����Թ�����ˮ�õ������ֲ�ƷΪ���ͼױ����ױ�����ʹ���Ը��������Һ��ɫ��
(3). ��ϩ����ϩ���Ժ�������Ȼ�̼��Һ�����ӳɷ�Ӧ��
(4). �����������̴�����������ռ����������Ǽ����������
(1). �۱�ϩ�����ϼ�ǿ��ʱ�õ��IJ����У���������������ϩ����ϩ�������ױ���̼���ɱ��зе����ݿ�֪�����Թ������ղ�������C����ʯ����Ҫ�ɷ���̼���ƣ�̼������ˮ��Ӧ�����������ƺ���Ȳ����ѧ����ʽΪ��CaC2 +2H2O �� Ca (OH)2 + C2H2�����ʴ�Ϊ��C��̼��CaC2 +2H2O �� Ca (OH)2 + C2H2����
(2). ���ݲ���ķе��֪����������ˮ��ȴ��õ��IJ�Ʒ�DZ��ͼױ���������ʹ���Ը��������Һ��ɫ���ױ�����ʹ���Ը��������Һ��ɫ���ʴ�Ϊ���ױ���
(3). �����г����IJ����к�����ϩ�ͱ�ϩ�����߶����Ժ�������Ȼ�̼��Һ�����ӳɷ�Ӧʹ������Ȼ�̼��Һ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��CH3CH=CH2��Br2��CH3CHBrCH2Br���ʴ�Ϊ����Һ��ɫ��CH2=CH2+Br2��CH2BrCH2Br��CH3CH=CH2��Br2��CH3CHBrCH2Br��
(4). �����������̴�����������ռ����������Ǽ�����������ʴ�Ϊ�����顢������

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�������̼����(Na2CS3)������ɱ��������������ijС�����ʵ��̽�������̼���Ƶ����ʲ��ⶨ����Һ��Ũ�ȡ�
ʵ��1��̽��Na2CS3������
���� | ���������� |
�� | ȡ����Na2CS3������������ˮ���Ƴ���Һ���ֳ����ȷ� |
�� | ������һ����Һ�еμӼ��η�̪��Һ����Һ���ɫ |
�� | ����һ����Һ�еμ�����KMnO4��Һ����ɫ��ȥ |
��1��H2CS3��________�ᣨ�ǿ������������
��2����֪����۵�����������SO42��,д���÷�Ӧ�����ӷ���ʽ______
��3��ijͬѧȡ�����������Һ���Թ��У��μ��������ᡢBaCl2��Һ������ɫ����������Ϊͨ���ⶨ�����İ�ɫ�����������������ʵ������Na2CS3���������Ƿ�ͬ�����Ĺ۵㲢˵������______��
ʵ��2���ⶨNa2CS3��Һ��Ũ��

����ͼ��ʾ���Ӻ�װ�ã�ȡ100mLNa2CS3��Һ����������ƿ�У�������d�Ļ�������������2.0mol/LϡH2SO4���رջ�����
��֪��Na2CS3 + H2SO4=Na2SO4 + CS2 + H2S����CS2��H2S���ж���CS2������ˮ���е�46�棬��CO2ijЩ�������ƣ���NaOH��������Na2COS2��H2O��
��4��ʢ����ˮCaCl2��������������______��B�з�����Ӧ�����ӷ���ʽ��______��
��5����Ӧ���������k���ٻ���ͨ����N2һ��ʱ�䣬��Ŀ����______��
��6��Ϊ�˼���Na2CS3��Һ��Ũ�ȣ���B�л������й��ˡ�ϴ�ӡ�������أ���19.2g���壬��A��Na2CS3�����ʵ���Ũ��Ϊ______��
��7����������ʵ�鷽����������ͨ���ⶨC����Һ����������ֵ������Na2CS3��Һ��Ũ�ȣ�����Ӧ������ͨ��N2��Ϊͨ�ȿ���������ֵ______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����