��Ŀ����
����Ŀ���л���W������������߷��Ӳ��Ϻϳɵ��м���ȣ��Ʊ�W��һ�ֺϳ�·�����¡�
��֪��![]()
��ش��������⣺
��1��F�Ļ�ѧ������_________���ڵķ�Ӧ������_________��
��2��D�к��еĹ�������________________��д���ƣ���D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ_____________��
��3����Ӧ�۵Ļ�ѧ����ʽ��______________________��
��4����Ӧ�Ļ�ѧ����ʽ��______________________��
��5�����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ
_______________��
��6�������л���W�������ϳ�·�ߣ������MΪ��ʼԭ���Ʊ�F�ĺϳ�·��(���Լ���ѡ)��[ʾ����![]() ]
]
____________________
���𰸡����״�ȡ����Ӧ�ǻ����Ȼ�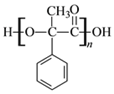

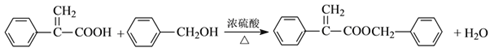
![]()
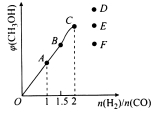
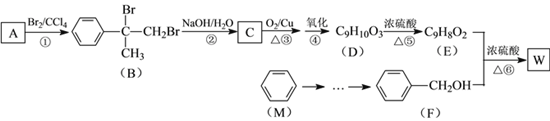
����������������ͼ��AΪ![]() ��CΪ
��CΪ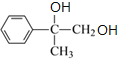 ��C�������IJ��������������D��DΪ
��C�������IJ��������������D��DΪ ��D��Ũ�������ʱ��ˮ����E��EΪ
��D��Ũ�������ʱ��ˮ����E��EΪ![]() ��E��F����������Ӧ����W��WΪ
��E��F����������Ӧ����W��WΪ![]() ��
��
(1)FΪ![]() �������DZ��״�����Ӧ����±������ˮ�ⷴӦ������ȡ����Ӧ���ʴ�Ϊ�����״���ȡ����Ӧ��
�������DZ��״�����Ӧ����±������ˮ�ⷴӦ������ȡ����Ӧ���ʴ�Ϊ�����״���ȡ����Ӧ��
(2)DΪ �����еĹ��������ǻ����Ȼ���D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ
�����еĹ��������ǻ����Ȼ���D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ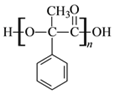 ���ʴ�Ϊ���ǻ����Ȼ���
���ʴ�Ϊ���ǻ����Ȼ��� ��
��
(3)��Ӧ���Ǵ��Ĵ���������Ӧ�Ļ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(4)��Ӧ������ʹ���������Ӧ����Ӧ�Ļ�ѧ����ʽΪ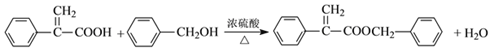 ���ʴ�Ϊ��
���ʴ�Ϊ��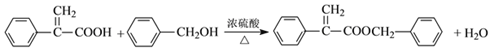 ��
��
(5)AΪ![]() �����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ
�����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ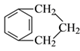 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(6)������Ϣ![]() �������ɱ��Ʊ��ױ����ױ����������� ����ʱ��������ȡ������ˮ�⼴�ɣ�����ͼΪ
�������ɱ��Ʊ��ױ����ױ����������� ����ʱ��������ȡ������ˮ�⼴�ɣ�����ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����г��ӣ������������ʣ���ѡ�õ��Լ���������������ȷ��һ���ǣ� ��
ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �������� |
A | CO2��HCl�� | ����NaHCO3��Һ | ϴ�� |
B | Mg��Al�� | ����ϡ���� | ���� |
C | FeCl2��Һ��FeCl3�� | ����Fe�� | ���� |
D | CO2��CO�� | O2 | ��ȼ |
A.A
B.B
C.C
D.D