��Ŀ����
����Ŀ��I��ijѧ����0.2000 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0������0���̶������£������¶���
����ȡ20.00mL����Һע����ϴ������ƿ�У�������1��2�η�̪��Һ
�ݵ���һ�α�Һ����Һ��ɫ����ɫ��Ϊ��ɫ����ֹͣ�ζ�����¼Һ�����
��ش�

��1�����ϲ����д�����ǣ����ţ�________��
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��______�С�����ͼ��ѡ������������������
��3�����в���������ʵ����ƫ����ǣ�______�����ţ�
A ��ʽ�ζ���δ��ϴ
B �ζ�ǰ���ζ��ܼ��������ݣ��ζ���������
C ��ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ
D �ζ�����ʱ���ӵζ��ܣ�����¼����
E �ζ���������һ�α�Һ�ɽ�����ƿ
��4���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע��_______________��
II.�����к͵ζ���ԭ�����ڹ�ҵ�����л����Խ���������ԭ�ζ��ⶨ���ʺ�����
��5��ˮ���иƾ������ò���Ƴ�����ϡH2SO4������,��![]() ����Һ�ζ�,ͨ���ⶨ��������ɼ�ӻ�֪�Ƶĺ���,�ζ���Ϊ:
����Һ�ζ�,ͨ���ⶨ��������ɼ�ӻ�֪�Ƶĺ���,�ζ���Ϊ:![]() .ʵ���г�ȡ0.400gˮ����Ʒ,�ζ�ʱ������0.0500 mol��L��1��
.ʵ���г�ȡ0.400gˮ����Ʒ,�ζ�ʱ������0.0500 mol��L��1��![]() ��Һ36.00 mL,���ˮ����Ʒ�иƵ���������Ϊ__________
��Һ36.00 mL,���ˮ����Ʒ�иƵ���������Ϊ__________
��6���ζ��յ��������__________��
���𰸡��٢ܢ� �� D��E ��ƿ����Һ��ɫ�仯 45.0�� �������һ�α�Һ������غ���ƿ����Һǡ������ɫ��Ϊ��dz����ɫ���Ұ���Ӳ��ָ�ԭ����ɫ
��������
��1���ٵζ���������ˮϴ�������ϴ������ϴ�ζ��ܣ���ʹ��Һ��Ũ�ȼ�С��
��2��������ʽ�ζ��ܣ����Ǽ�ʽ�ζ��ܣ�
��3��A����ƿ��ˮ����Ӱ��ζ������
B����ʽ�ζ��ܼ��������ݣ����ĵı�Һ�����ƫ�ߣ�
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ����Ӱ�죻
D������ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ������ҺƫС��Ũ��ƫ�ͣ�
��4���ζ�����Һ����ɫ���dz��ɫʱ���Ұ����֮�ڲ��ٸı䣬����ζ��յ㣻
��5�����ݹ�ϵʽ5Ca2+��5H2C2O4��2KMnO4���м��㣻
��6���ζ��յ��������Թ�������ɫ������Һ����ɫ��Ϊ��dz����ɫ��
��1���ٵζ���������ˮϴ����ϴ��ʹ��Һ��Ũ�ȼ�С�����ĵ�������ⶨ���ƫ�����Ա���������������Һ��ϴ���ʢٲ�������
�����ô���Һ��ϴ��ƿ����ʹ����Һ�������ʵ������ӣ����ı�Һ��������ⶨ���ƫ��������ƿ������ϴ���ʢܲ�������
�ݵ���һ�α�Һ����Һ��ɫ����ɫ��Ϊ��ɫ����ֹͣ�ζ�����ʱδ���յ㣬Ӧ����ɫ��Ϊ��ɫ�Ұ�����ڲ��ٸı䣬�ζ��������ʢݲ�������
�ʴ�Ϊ���٢ܢݣ�
��2����������Ӧ���ü�ʽ�ζ��ܣ���ѡ�ң�
��3������c�����⣩=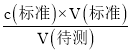 ��������������V��������Ӱ�죺
��������������V��������Ӱ�죺
A����ʽ�ζ���δ��ϴ������Һ��ϡ�ͣ�Ũ�ȱ�С�����ı�Һ�������С��ʹ�ζ����ƫС��ѡ��A������
B���ζ�ǰ���ζ��ܼ��������ݣ��ζ��������ݣ����ĵı�Һ�������С��ʹ�ζ����ƫС��ѡ��B������
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ����Ӱ��ζ������ѡ��C������
D���ζ�����ʱ���ӵζ��ܣ�����¼���ݣ�ʹ��Һ�����ƫ��ʹ�ζ����ƫ��ѡ��D��ѡ��
E���ζ���������һ�α�Һ�ɽ�����ƿ�����ĵı�Һ���������ʹ�ζ����ƫ��ѡ��E��ѡ��
��ѡDE��
��4���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��
�ʴ�Ϊ����ƿ����Һ��ɫ�仯��
��5����Ӧ�Ĺ�ϵʽΪ5Ca2+��5H2C2O4��2KMnO4��
n(KMnO4)=0.0500mol/L��36.00mL=1.80mmol��
n(Ca2+)=4.50mmol��
ˮ���иƵ���������Ϊ![]() ��100%=45.0%��
��100%=45.0%��
��6���ζ��յ�������ǵ������һ�α�Һ������غ���ƿ����Һǡ������ɫ��Ϊ��dz����ɫ���Ұ���Ӳ��ָ�ԭ����ɫ��

����Ŀ����2L�ܱ������ڣ�����0.100molCO�����0.080molCuO���壬800��ʱ�������·�Ӧ��2CuO��s��+CO��g��![]() Cu2O��s��+CO2��g����n��CuO����ʱ��ı仯�����
Cu2O��s��+CO2��g����n��CuO����ʱ��ı仯�����
ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��CuO����mol�� | 0.080 | 0.060 | 0.040 | 0.020 | 0.020 | 0.020 |
��1����CO��ʾǰ2min�ڵĻ�ѧ��Ӧ����=_______
��2������˷�Ӧ��800Cʱ�Ļ�ѧƽ�ⳣ��K=_______
��3������ƽ������ϵ�м���CO��CO2��0.05mol�����ʱV������_______V���棩
��4��������ԭCuO��CO������C��ˮ������Ӧ�Ƶá�
��֪��C��s����O 2��g��= CO2��g�� H=-393.5kJ/mol
2CO(g)+O2(g)=2CO2(g) H=-566kJ/mol
2H2(g)+O2(g)=2H2O(g) H=-571.6kJ/mol
��C��s����H2O��g��![]() CO��g����H2��g�� H= __________��
CO��g����H2��g�� H= __________��
����Ŀ����ҵ����ȡCuCl2�������������£�
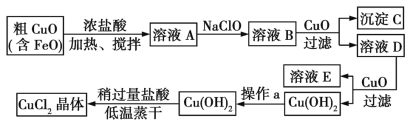
�����±����ݣ��ش����⣺
���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
�ܶȻ�(25 ��) | 8.0��10-16 | 2.2��10-20 | 4.0��10-38 |
��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3~4 |
(1)����ҺA�м���NaClO��Ŀ����_________________��
(2)����ҺB�м���CuO��Ҫ�漰�����ӷ�Ӧ����ʽΪ________________��
(3)����aΪ___________��
(4)��Cu(OH)2�м�������ʹCu(OH)2ת��ΪCuCl2�������Թ�������͵������ɵ�Ŀ����___��