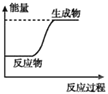
��Ŀ����
����Ŀ���ش���������:
(1)��֪������CO��ȼ����Ϊ283kJ/mol����CO��ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
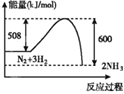
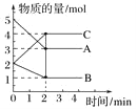
(2)��ҵ������CO��H2�ϳ������ԴCH3OH���䷴ӦΪ:CO(g)+2H2(g)![]() CH3OH(g) H=-116kJ/mol����ͼ�ٱ�ʾCO��ƽ��ת����(
CH3OH(g) H=-116kJ/mol����ͼ�ٱ�ʾCO��ƽ��ת����(![]() )���¶Ⱥ�ѹǿ�仯��ʾ��ͼ�к�����X��ʾ����_________��Y1______Y2(������������=����������)��
)���¶Ⱥ�ѹǿ�仯��ʾ��ͼ�к�����X��ʾ����_________��Y1______Y2(������������=����������)��
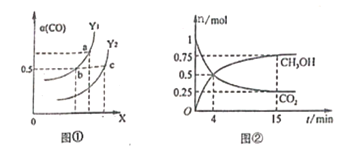
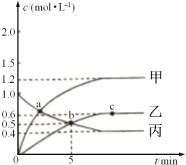
(3)�ϳɼ״��ķ�Ӧԭ��Ϊ:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����0.5L���ܱ����У�����1 mol CO2��3molH2����500��������Ӧ�����CO2(g��CH3OH(g)������ʱ��仯��ͼ����ʾ��
CH3OH(g)+H2O(g)����0.5L���ܱ����У�����1 mol CO2��3molH2����500��������Ӧ�����CO2(g��CH3OH(g)������ʱ��仯��ͼ����ʾ��
�ٷ�Ӧ���е�4minʱ��v(��)____v(��)(������������=����������)��0��4min��H2��ƽ����Ӧ����v(H2)=_____________��
�ڸ��¶���ƽ�ⳣ��Ϊ_____________��
��������˵���÷�Ӧ�Ѵﵽƽ��״̬����___________��
A. v��(CH3OH)=3v��(H2)
B.CO2��H2��CH3OH��H2OŨ��֮��Ϊ1:3:1:1
C.���º�ѹ�£������������ٱ仯
D.���º����£�������ܶȲ��ٱ仯
(4)Ϊ���ȼ�ϵ����������ʣ����������Ϊȼ�ϵ�ء�ij����Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ���Ծ��д����ú͵������ܵ�ϡ������Ϊ�缫д����ȼ�ϵ�صĸ�����Ӧʽ��_________��
���𰸡�CO(g)+ ![]() O2(g) = CO2(g) ��H=-283kJ/mo1 ѹǿ �� �� 0.75 mol��L1��min1
O2(g) = CO2(g) ��H=-283kJ/mo1 ѹǿ �� �� 0.75 mol��L1��min1 ![]() C CH3OH - 6e- + 8 OH- = CO32-+6H2O
C CH3OH - 6e- + 8 OH- = CO32-+6H2O
��������
(1)����CO��ȼ����Ϊ283kJ/mo1�����ȼ���ȵĻ�ѧ����ʽ����дҪ����д��
(2)CO(g)+2H2(g)CH3OH(g)��H=-116kJ/mo1����Ӧ�����������С�ķ��ȷ�Ӧ��ͼ����CO ��ƽ��ת������X�����������ͬ������Y1��Y2������һ����̼ת����Y1��Y2������¶Ⱥ�ѹǿ��ƽ���Ӱ������жϣ�
(3)��ͼ�����4minʱ���״��Ͷ�����̼Ũ����ʱ�������仯����Ӧδ�ﵽƽ��״̬������ͼ���ȼ���v(CO2)���ٸ��ݷ���ʽ����v(H2)����15min��Ӧ�ﵽƽ��״̬������ƽ��ʱ�״������ʵ���Ϊ0.75mol���������ʽ����ƽ�ⳣ��K���۸������淴Ӧ������ͬ������ֺ������ֲ�������жϣ�
(4)��������������Ӧ���״�����������̼��أ��ݴ���д�缫��Ӧʽ��
(1)CO��ȼ����Ϊ283kJ/mo1����ȼ���ȵ��Ȼ�ѧ����ʽΪCO(g)+![]() O2(g)CO2(g)��H=-283kJ/mo1���ʴ�Ϊ��CO(g)+
O2(g)CO2(g)��H=-283kJ/mo1���ʴ�Ϊ��CO(g)+![]() O2(g)CO2(g)��H=-283kJ/mo1��
O2(g)CO2(g)��H=-283kJ/mo1��
(2)CO(g)+2H2(g)CH3OH(g)��H=-116kJ/mo1���÷�Ӧ�����������С�ķ��ȷ�Ӧ��ͼ����CO ��ƽ��ת������X�����������X ��ʾ����ѹǿ��Y��ʾ�����¶ȣ���ͬ�����£���Y1��Y2������һ����̼ת����Y1��Y2�������¶ȣ�ƽ�������ƶ������¶�Y1��Y2���ʴ�Ϊ��ѹǿ������
(3)�ٷ�Ӧ���е�4min ʱ����ʱ��仯���״����ӣ�������̼��С��˵����Ӧ����������У�v(��)��v(��)��0��4min��������̼���ʵ����仯1mol-0.5mol=0.5mol��CO2��ƽ����Ӧ����v(CO2)= =0.25mol/(Lmin)����v(H2)=3 v(CO2)=3��0.25mol/(Lmin)=0.75mol/(Lmin)���ʴ�Ϊ������0.75 mol/(Lmin)��
=0.25mol/(Lmin)����v(H2)=3 v(CO2)=3��0.25mol/(Lmin)=0.75mol/(Lmin)���ʴ�Ϊ������0.75 mol/(Lmin)��
��15min��Ӧ�ﵽƽ��״̬���״�ƽ��Ũ��Ϊ0.75mol/L��������̼ƽ��Ũ��Ϊ0.25mol/L��
CO2(g)+3H2(g)CH3OH(g)+H2O(g)
��ʼ��(mol) 1 3 0 0
�仯��(mol) 0.75 2.25 0.75 0.75
ƽ����(mol) 0.25 0.75 0.75 0.75
K= =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��A������֮�ȵ��ڻ�ѧ����ʽ�еĻ�ѧ������֮�ȣ�3v��(CH3
(4)ȼ�ϵ���У�ͨ��ȼ�ϵ�Ϊ��������������������Ӧ���״�����������̼��أ��缫����ʽΪCH3OH - 6e- + 8 OH- = CO32-+6H2O���ʴ�Ϊ��CH3OH - 6e- + 8 OH- = CO32-+6H2O��