��Ŀ����
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����±���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к�������Ԫ�ء�
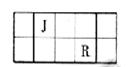
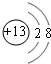
��1��Ԫ��T�����ڱ���λ�ڵ�_________�塣
��2��J������ɵĻ�����Aÿ��������4��ԭ����ɣ���ṹ��ʽΪ________________����֪���ȼ��a gA����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ����A����ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________��
��3��M��R�γɵ�һ�ֻ�������ʹ���Ը��������Һ��ɫ���÷�Ӧ�����ӷ���ʽΪ___________________________________________________��
��4�������ӹ�ҵ�У�L�������̬�⻯���ˮ��Һ������ʴ��H2O2 �����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ��
___ __ _��
��5��д�����ֽ�����������Ԫ���е�һ�ֻ�����Ԫ���γɵ�Ư���Ļ�ѧʽ��______________________��
��6������L��M��R�γɵ��⻯��е��ɸߵ��͵�˳����______________________���û�ѧʽ��ʾ��
��1���ڢ�A �塣
��2�� CH��CH ��C2H2(g)��5/2O2(g)��2CO2(g)��H2O(l)�� ��H����2b kJ / mol
��3��2MnO4�� + 5SO2 + 2H2O = 2Mn2+ +5SO42- + 4H+
��4��2NH3 + 3H2O2 = N2 + 6H2O
��5�� O3��Cl2��SO2��ClO2 �����
��6�� H2O> NH3> H2S
����:
��

 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 Al��OH��3+3HCl
Al��OH��3+3HCl
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�