��Ŀ����
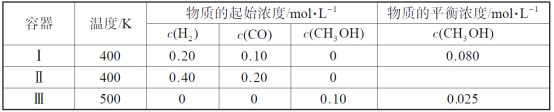
����Ŀ��(1)��״����VL�����ܽ���1Lˮ��(ˮ���ܶȽ���Ϊ1g/mL),������Һ���ܶ�Ϊ��g/mL,�����Һ�����ʵ����ʵ���Ũ��Ϊ______��
(2)��11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4��,ÿ1molCuSO4�������������ʵ�����______��
(3)����ͬ�ݻ����ܷ�����A��B,������,A�г���agA����,B�г���agCH4����,A��B�ڵ�ѹǿ֮����4:11,��A��Ħ������Ϊ______��
(4)20mLCaCl2��Һ��ˮϡ����100mL,ϡ�ͺ����Һ��Cl-���ӵ����ʵ���Ũ��Ϊ1mol/L,��ϡ��ǰCaCl2�����ʵ���Ũ��Ϊ______��
(5)��һ���¶Ⱥ�ѹǿ��,3L����A2��9L���������B2��ȫ��������6L���ij����C,�������C�Ļ�ѧʽΪ(��A��B��ʾ)______��
���𰸡�![]() 1/5 44g/mol 2.5mol/L AB3��B3A
1/5 44g/mol 2.5mol/L AB3��B3A
��������
��1����״����VL���������ʵ���Ϊ![]() mol������Ϊ
mol������Ϊ![]() mol��17g/mol=
mol��17g/mol=![]() g���ܽ���1Lˮ�У�������Һ������Ϊ
g���ܽ���1Lˮ�У�������Һ������Ϊ![]() g+1000g�����Ϊ��V=
g+1000g�������V=![]() =
=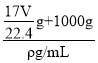 =
=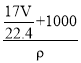 mL����
mL����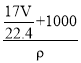 ��103L������Һ�����ʵ����ʵ���Ũ��Ϊ��c=
��103L������Һ�����ʵ����ʵ���Ũ��Ϊ��c=![]() =
= =
=![]() mol/L��
mol/L��
��2����11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4�У�P��Cu3P�У�PԪ�ػ��ϼ۴�0�۽���Ϊ-3�ۣ�P��H3PO4��PԪ�ػ��ϼ۴�0������Ϊ+5�ۣ�CuԪ�ش�+2�۽���Ϊ+1�ۣ��ɷ���ʽ��֪��CuSO4���ϼ۽�������Ϊ15��15mol CuSO4������3molP����1molCuSO4�������������ʵ�����![]() mol=
mol=![]() mol��
mol��
��3��������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȣ���A��Ħ������ΪM�����У�![]() =4��11����֮��M=44g/mol��
=4��11����֮��M=44g/mol��
��4����ϡ��ǰCaCl2�����ʵ���Ũ��Ϊx��ϡ��ǰ�����ӵ����ʵ������䣬��0.02L��x��2=0.1L��1mol/L�����x=2.5mol/L��
��5��ͬһ�����£��μӷ�Ӧ����������֮�ȵ����������֮�ȣ���A2��B2��C�ļ�����֮��=3L��9L��6L=1��3��2���÷���ʽΪA2+3B2=2C������ԭ���غ�֪��C�Ļ�ѧʽΪAB3��B3A��

����Ŀ����ͼ��ʵ�����Ʊ���������֤�������ʵ�װ��(���мг�װ����ʡ��)��
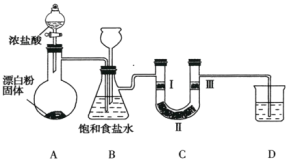
��֪��װ��A�������ķ���װ�ã���Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
�ݴ˻ش��������⣺
(1)װ��B�б���ʳ��ˮ��������_________��
(2)װ��BҲ�ǰ�ȫƿ��Ŀ���Ǽ��ʵ�����ʱװ��C���Ƿ�����������д��װ��C�з�������ʱװ��B�е�ʵ������__________________��
(3)װ��C����������֤�����Ƿ����Ư���ԣ���װ��C��I��������Ӧ�����������___________(����ĸ)��
��� | I | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
(4)װ��D��������_____________________________��
(5)�����20mL��10mol��L-1��Ũ����������������Ƴ�ַ�Ӧ��ʵ�����ռ����������ڱ�״���µ������__________��
A.��2.24 L B.��2.24 L C.��2.24 L D.��2.24 L