��Ŀ����
15���ϳɻ�����II��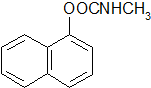 �����������£�
�����������£�
��1��������I�ķ���ʽΪC2H5ON������2�ķ�Ӧ������������Ӧ��
��2�����黯����II�п��ܻ������ӣ�
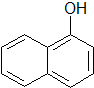 ����������Լ����Ȼ�����Һ��Ũ��ˮ��T�棬a mol/L������Һ��pH=b������¶������ӵĵ���ƽ�ⳣ��K=$\frac{1{0}^{-2b}}{a-1{0}^{-b}}$��
����������Լ����Ȼ�����Һ��Ũ��ˮ��T�棬a mol/L������Һ��pH=b������¶������ӵĵ���ƽ�ⳣ��K=$\frac{1{0}^{-2b}}{a-1{0}^{-b}}$����3���״�������CH3NH2�����ʣ���CO��һ��������Ҳ�ܽ��в���1��Ӧ���ɻ������IV�Ǣ��ͬ���칹�壬ˮ��Һ�����ԣ�IV�Ľṹ��ʽ��CH3COOH��
��4���йػ�����II������˵����ȷ����CD��
A��������II��һ�ַ�����
B����������1mol������II�������6mol H2�ӳ�
C��������II�˴Ź���������9���
D��������II�ܽ���ȡ�����ӳɷ�Ӧ��
���� �ɺϳ����̿�֪������1��N-H���Ѳ���C=O������2Ϊ����������3Ϊȡ����Ӧ��
��1���ɽṹ��֪����ʽ������2��ȥH��O��
��2�����Ӻ���-OH����Ϸӵ����ʼ��飻T�棬a mol/L������Һ��pH=b����c��H+��=10-bmol/L�����ӵ�Ũ��Ϊ��a-10-b��mol/L��
��3���״�������CH3NH2�����ʣ���CO��һ���������ܽ��в���1��Ӧ���ɻ������O-H�����ѣ������ΪCH3OOCH��IV�Ǣ��ͬ���칹�壬ˮ��Һ�����ԣ���-COOH��
��4��������II��������-COOC-��-CONH-����ϱ��������ļ������������
��� �⣺��1���ɽṹ��֪����ʽΪC2H5ON������2��ȥH��O��Ϊ������Ӧ���ʴ�Ϊ��C2H5ON��������Ӧ��
��2�����Ӻ���-OH����ѡ���Ȼ�����Һ��Ũ��ˮ���飻T�棬a mol/L������Һ��pH=b����c��H+��=10-bmol/L�����ӵ�Ũ��Ϊ��a-10-b��mol/L������¶������ӵĵ���ƽ�ⳣ��K=$\frac{��1{0}^{-b}��^{2}}{��a-1{0}^{-b}��}$=$\frac{1{0}^{-2b}}{a-1{0}^{-b}}$���ʴ�Ϊ���Ȼ�����Һ��Ũ��ˮ��$\frac{1{0}^{-2b}}{a-1{0}^{-b}}$��
��3���״�������CH3NH2�����ʣ���CO��һ���������ܽ��в���1��Ӧ���ɻ������O-H�����ѣ������ΪCH3OOCH��IV�Ǣ��ͬ���칹�壬ˮ��Һ�����ԣ���-COOH��IV�Ľṹ��ʽ��CH3COOH���ʴ�Ϊ��CH3COOH��
��4��������II��������-COOC-��-CONH-����
A��������II��O��NԪ�أ�����һ�ַ���������A����
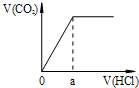
B��ֻ�л�״�ṹ�в����ͼ������������ӳɷ�Ӧ�����������1mol������II�������5mol H2�ӳɣ���B����
C���ṹ���Գƣ���9��λ�õ�H������II�˴Ź���������9��壬��C��ȷ��
D��������II��-COOC-��-CONH-�ܽ���ȡ�����������ܷ����ӳɷ�Ӧ����D��ȷ��
�ʴ�Ϊ��CD��
���� ���⿼���л���ĺϳɼ��ṹ�����ʣ�Ϊ��Ƶ���㣬���պϳɷ�Ӧ�е���Ϣ���ϳɷ�ӦΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д��ٻ�ѧ��ֻ���ڷ���֮��
�����ӻ������в����ܺ��Ǽ��Լ�
�۹��ۻ������в����ܺ����Ӽ�
���ɷǽ���Ԫ���γɵĻ����ﶼ�ǹ��ۻ�����
��ǿ�����һ�������ӻ�����
���ۻ���������Ƿǵ����
���ȶ��ԣ�H2O��H2S��������йأ�
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | ����NaNO3���壬v��H2����С | B�� | 20mL BaCl2��Һ��v��H2������ | ||
| C�� | ����NaHSO4���壬v��H2������ | D�� | ����18mol/L�����ᣬv��H2������ |
| �� | �� | �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
��1���۵�Ԫ�ط�����Li���������������Ӧˮ������Al��OH��3��
����Ԫ�����ڱ��е�λ���ǣ����ڡ��壩�ڶ����ڢ�A�壮
��2��������ǿ�Ļ�����ĵ���ʽ�ǣ�
 �������ӻ��������ӡ����ۡ�����
�������ӻ��������ӡ����ۡ�������3���ȽϢܺ͢ߵ��⻯����ȶ��ԣ��û�ѧʽ��ʾ��NH3��PH3��
��4��д���ߵ�����������Ӧˮ����������⻯�ﷴӦ�Ļ�ѧ����ʽ��NH3+HNO3=NH4NO3��
��5��д��������������Ӧˮ������ݵ��⻯��ˮ��Һ��Ӧ�����ӷ���ʽ��Mg��OH��2+2H+=Mg2++2H2O��
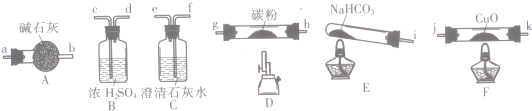
��ش�
��1������������װ�ô����ҵ�����˳��Ϊ��i��cd��gh��hg��ab��jk��kj��feβ���������������ӿڵ���ĸ��ţ���
��2��˵��CO�ܻ�ԭCuO��ʵ������ΪF��Ӳ�ʲ������еĺ�ɫ�����Ϊ��ɫ��C������ʯ��ˮ����ǣ�
��3����ʵ�鴦��β���ķ���Ϊ��ȼ�յ����������ռ���
��4����ȥ��Bװ�ã����ܲ�����Ӱ��ΪNaHCO3�ֽ������ˮ������������̼�۷�Ӧ����H2����ʵ��������ţ�
��5������װ�ð���ȷ��˳�����Ӻ����ʵ�飮����Ӧ������F��Ӳ�ʲ������еĹ���ȫ����Ϊ��ɫ��
[��������]Cu��Cu2O��Ϊ��ɫ��Cu2O��������Һ��������Cu��Cu2+��
[�������]��ɫ����ijɷֿ���Ϊ����Cu����Cu2O����Cu��Cu2O��
[ʵ����֤]��С��Ϊ��֤�������룬�ֱ�ȡ������ɫ��������Թ��У���������ʵ�飮
| ʵ���� | ���� | ���� |
| a | ��������ϡ���ᡢ�� | �Թ��ں�ɫ������ȫ�ܽ⣬��Һ��Ϊ��ɫ��������ɫ������� |
| b | ��������ϡ���ᡢ�� | �Թ����к�ɫ���壬��ҺΪ��ɫ |
[ʵ�����]��������ʵ��������֪��ɫ����ijɷ�ΪCu���ѧʽ����
| A�� | ������Һ����ǿ�����ԣ���������ˮ�� | |
| B�� | �������費���κ��ᷴӦ������ʯӢ������������ | |
| C�� | ͭ�Ľ�����Ա����������ں��������װ����ͭ���Լ����丯ʴ | |
| D�� | �����£����ܱ�Ũ����ۻ����������Ʋ۳�����Ũ���� |
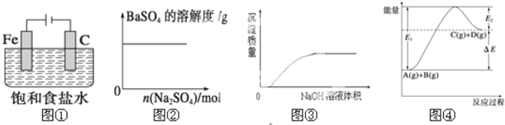
| A�� | ͼ�ٱ�ʾ��ֹ����ʴ��װ��ͼ | |
| B�� | ͼ�ڱ�ʾ��BaSO4������Һ�м��������� | |
| C�� | ͼ�۱�ʾ��NaOH��Һ����Ba��HCO3��3��Һ�� | |
| D�� | ͼ�ܱ�ʾ��Ӧ����ܼ��ܴ�����������ܼ��� |