��Ŀ����
����Ŀ��ʵ��������240 mL1.0mol��L��1��NaOH��Һ��ʵ����������У�
A.���ձ�����ƽ�ϳƳ�ag�������ƹ��壬������������ˮʹ����ȫ�ܽⲢ��ȴ�����£�
B.����������ƿ�м�����ˮ��Һ���̶���1��2 cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ����̶������У�
C.���Ƶõ���ҺС��ת��������ƿ�У�
D.������ƿƿ�����������ҡ�ȡ�
E.����������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ע������ƿ��������
����д���пհף�
��1�������������ȷ˳��Ϊ_____������ĸ����
��2����ʵ������õ�����������ƽ��ҩ�ס����������ձ���____��____��
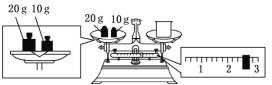
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��ʾ������������ձ���ʵ������Ϊ___g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�___gNaOH��
��4��ʹ������ƿǰ������е�һ��������____��
��5�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���___��
A.����ֽ����NaOH����
B.����ʱ���ӿ̶���
C.δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
D.���ݺ�����ƿ��������תҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶���
��6����������ˮ����ʱ���������̶��ߣ�����������_____��
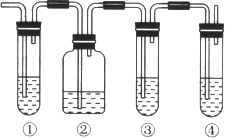
���𰸡�ACEBD 250mL����ƿ ��ͷ�ι� 27.4 10.0 ��© B C ��������
��������
(1)����ʵ������IJ��裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ����Լ�ÿ��������Ҫ����ȷ����Ӧ���������������(1)��(2)��
(3)����ͼʾ���ձ�������+2.6g=30g��ʵ��������240 mL1.0mol��L��1��NaOH��Һ����Ҫѡ��250mL����ƿ���ƣ��ݴ˷������
(4)����ƿ�ڲ������ӣ�ʹ��ǰ�����©��
(5)����c=![]() ��������ʵ����ʵ���n����Һ�����V�ı仯������������
��������ʵ����ʵ���n����Һ�����V�ı仯������������
(6)ʵ�����д�����Ҫ����������Һ��
(1)����240 mL 1.0mol��L��1��NaOH��Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ����Բ����������ȷ˳��Ϊ��ACEBD���ʴ�Ϊ��ACEBD��
(2)ʵ��IJ��������У����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ�ù���ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
(3)����ͼʾ���ձ�������+2.6g=30g�����ձ�������Ϊ27.4g��ʵ��������240 mL1.0mol��L��1��NaOH��Һ����Ҫѡ��250mL����ƿ������250 mL��Һ����ҪNaOH������=0.25L��1.0mol��L��1��40g/mol=10.0g���ʴ�Ϊ��27.4��10.0��
(4)�����ӵ�����ʹ��ǰҪ�Ȳ�©��������ƿʹ��ǰ�����©���ʴ�Ϊ����©��
(5)A���������ƹ������׳��⣬����ֽ����NaOH���壬ʹ���������Ƶ�����ƫС�������Ƶ���Һ��Ũ��ƫ�ͣ���A����
B������ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���B��ȷ��
C��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ������ȴ����Һ���ƫС��Ũ��ƫ�ߣ���C��ȷ��
D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶����������ģ��ټ�ˮ���̶��ᵼ��Ũ��ƫ�ͣ���D����ѡBC��
(6)��������ˮ����ʱ���������̶��ߣ���ȷ�Ĵ��������ǣ�������ƿϴ�Ӹɾ����������ƣ��ʴ�Ϊ���������ơ�