��Ŀ����
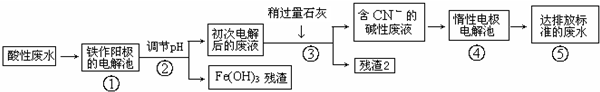
1���ڵ����µ�ⱥ��KHSO4��Һ���Ʊ�K2S2O8�����װ����ͼ��ʾ������˵������ȷ���ǣ�������
| A�� | �����ĵ缫��ӦʽΪ2SO42--2e-�TS2O82- | |
| B�� | �����ܷ�Ӧ����ʽΪ2KHSO4$\frac{\underline{\;ͨ��\;}}{\;}$K2S2O8+H2�� | |
| C�� | һ��ʱ�����Һ��pH��С | |
| D�� | �������У�������������ʹʪ��ĵ���KI��ֽ��������ɫ�������壬�����������O3 |
���� A�����KHSO4��Һ��SO42-ʧ��������S2O82-���ӵ�ʧ�����غ��֪2molSO42-ʧȥ2mol���ӣ�
B���ý�������أ�������������ŵ磬�������������ŵ磻
C�����ݵ缫��Ӧ����ʽ�ж�������Ũ�ȼ�С��
D������Ϊ�����ӷŵ磬����������I-�����壬��Ϊ��ɫ����ӦΪO3���������OH-�ŵ�����O2��O2�ڷŵ������½�һ��ת��ΪO3��
��� �⣺A�����KHSO4��Һ��SO42-ʧ��������S2O82-�����ϼ۱仯Ϊ��+6������Ϊ+7�ۣ�ʧȥ1�����ӣ��ʵ缫��ӦʽΪ��2SO42--2e-�TS2O82-����A��ȷ��
B�����KHSO4��Һ��SO42-ʧ��������S2O82-��H+�õ�������H2����Ӧ�Ļ�ѧ����ʽΪ��2KHSO4$\frac{\underline{\;ͨ��\;}}{\;}$K2S2O8+H2������B��ȷ��
C��ͨ��B������֪��2KHSO4$\frac{\underline{\;ͨ��\;}}{\;}$K2S2O8+H2�����ʷ�Ӧһ��ʱ�����Һ��������Ũ�ȼ��٣�pH����C����
D������Ϊ�����ӷŵ磬����������I-�����壬��Ϊ��ɫ����ӦΪO3���������OH-�ŵ�����O2��O2�ڷŵ������½�һ��ת��ΪO3����D��ȷ��
��ѡC��
���� ���⿼���˵��ԭ���ķ����жϣ�ʵ����̷����жϣ������������ʺ͵��ԭ����������ԭ��Ӧ�����غ���ƽ���ӷ���ʽ�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�| ������0.1g�� | ����̿ | FeCl3 | KI | MnO2����״ | MnO2��ĩ״ |
| ǰ15s��������������ml��[�� | 5 | 11 | 7 | 8[ | 11 |
| ǰ30s��������������ml�� | 8 | 16 | 11 | 11 | 21 |
| ǰ45s��������������ml�� | 11 | 20 | 15 | 18 | 35 |
| ǰ60s��������������ml�� | 15 | 24 | 20 | 26 | 49 |
��2�����ϱ����Եó�������̿��FeCl3��KI ��MnO2����ĩ״�����ִ����У���Ч���ɴ�С��˳����MnO2����ĩ״����FeCl3��KI������̿���������Ĺ��������������С�Դ�Ч����ʲôӰ�����ԽС��Ч��Խ�ã�
��3��ʵ�鷢�����ʵĶ�������H2O2 �ķֽ�Ҳ�������Ĵ����ã���������Һ�м������ᣬ�����Һ����ʱ���ͻ�ʧȥ�������������Ľ����Ǵ���ʧȥ���ԣ�
| A�� | �屽��ˮ | B�� | �������Ҷ��� | C�� | ����Ҵ� | D�� | ���ͺ�ˮ |
| A�� | 2H2O2 $\frac{\underline{\;MnO_2\;}}{\;}$ 2H2O+O2�� | |
| B�� | NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+HCl�� | |
| C�� | 2Al+Fe2O3 $\frac{\underline{\;����\;}}{\;}$ 2Fe+Al2O3 | |
| D�� | Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O |




 ��
��