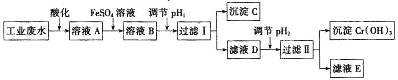
��Ŀ����
����Ŀ��������Ҫ�Ĺ�ҵԭ�ϣ���ũҵ��ҽҩ�������ͻ�������������ҪӦ�á�
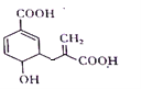
��1�������ĵ���ʽΪ___�����Ĺ��ۼ�����___(����ԡ��Ǽ��ԡ�)����������ˮ�Լ��Ե������ǣ��û�ѧ����ʽ��ʾ��___��
��2����ҵ����N2��H2��һ�������ºϳɰ������д�ʩ��ʹ����Ӧ����������һ��ʹƽ��������NH3����������������___��
A�����ͷ�Ӧ�¶� B��ѹ����Ӧ����� C������N2 D��Һ������NH3
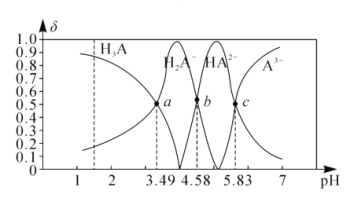
��3�������£���100mL0.2mol/L�İ�ˮ����μ���0.2mol/L�����ᣬ������Һ��pH����Һ��NH4+��NH3��H2O�����ʵ���������������������Ĺ�ϵ��ͼ��ʾ��
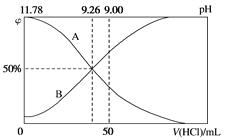
��ʾNH3��H2OŨ�ȱ仯��������___(�A����B��)��
��4���������������Ϊ50mLʱ����Һ��c(NH4+)��c(NH3��H2O)=___mol/L(�����ֱ�ʾ)����Һ����Ҳ��������ˮ�ĵ���(H2O��H2O![]() H3O����OH��)��̼��������Һ����Ҳ�ܷ�����ȫ���������ˮ��İ��⡣
H3O����OH��)��̼��������Һ����Ҳ�ܷ�����ȫ���������ˮ��İ��⡣
��д��Һ���ĵ��뷽��ʽ��___��
��д��̼��������Һ�����һ����������ӷ���ʽ��___��
��д��̼���Ƶ�Һ����Һ�и�����Ũ�ȵĴ�С��ϵ��___��
���𰸡� ����
���� ![]() ��
��![]() B A 2��10-5-2��10-9
B A 2��10-5-2��10-9 ![]()
![]() c��Na+����c��CO32-����c��NH2-����c��NH4CO3-����c��NH4+��
c��Na+����c��CO32-����c��NH2-����c��NH4CO3-����c��NH4+��
��������
��1�������ĵ���ʽΪ �����Թ��ۼ��Dz�ͬԪ��֮���γɵĹ��ۼ����Ǽ��Թ��ۼ���ͬ��Ԫ��֮���γɵĹ��ۼ�����������ˮ����һˮ�ϰ���
�����Թ��ۼ��Dz�ͬԪ��֮���γɵĹ��ۼ����Ǽ��Թ��ۼ���ͬ��Ԫ��֮���γɵĹ��ۼ�����������ˮ����һˮ�ϰ���
��2����ҵ����N2��H2��һ�������ºϳɰ��ķ�Ӧ�����������С�ķ��ȷ�Ӧ��
��3����100mL0.2mo/L�İ�ˮ����μ���0.2mol/L�����ᣬһˮ�ϰ�Ũ�ȼ�С��
��4�����ݵ���غ�������غ���㣻
��Һ����ˮ�������ƣ��ݴ�д�����˷���ʽ��
������ˮ���ʵ���������ӽ��ˮ������������ӻ������������γ�������ʵĹ��̣�
�۰�����ڵ����ӷ���ʽΪ��![]() ��
��![]() ���ݴ˷����ж�����Ũ�ȴ�С��
���ݴ˷����ж�����Ũ�ȴ�С��
��1�������ĵ���ʽΪ ��N-H���Dz�ͬԪ���γɵĹ��ۼ�����Ϊ���Թ��ۼ�����������ˮ����һˮ�ϰ���һˮ�ϰ�Ϊ����ʴ�Ϊ��
��N-H���Dz�ͬԪ���γɵĹ��ۼ�����Ϊ���Թ��ۼ�����������ˮ����һˮ�ϰ���һˮ�ϰ�Ϊ����ʴ�Ϊ�� �����ԣ�
�����ԣ�![]() ��
��![]() ��
��
��2��A�����ͷ�Ӧ�¶ȣ�ƽ��������У�����Ӧ���ʼ�С����A����
B��ѹ����Ӧ���������ѹǿ��Ӧ��������ƽ��������У������������һ������B��ȷ��
C������N2 ��������ƽ��������У������������������һ������C����
D��Һ������NH3 ƽ��������У���Ӧ���ʼ�С����D����
�ʴ�Ϊ��B��
��3����������100mL0.2mo/L�İ�ˮ����μ���0.2mol/L�����ᣬһˮ�ϰ�Ũ�ȼ�С����ʾNH3H2OŨ�ȱ仯��������A���ʴ�Ϊ��A��
��4���������������Ϊ50mlʱ����ʱ��ҺpH=9����Һ�д��ڵ�Ũ�ȵ�һˮ�ϰ����Ȼ�泥���Һ�д��ڵ���غ�c��NH4+��+c��H+��=c��Cl-��+c��OH-���������غ�õ���c��NH4+��+c��NH3H2O��=2c��Cl-�����õ�c��NH4+��-c��NH3H2O��=2c��OH-��-2c��H+��=2��10-5-2��10-9���ʴ�Ϊ��2��10-5-2��10-9��
����Һ����Ҳ��������ˮ�ĵ��룬��Һ���ĵ��뷽��ʽΪ��![]() ��
��
��̼������ǿ�������Σ�̼������ӽ��Һ��������������ӷ������⣬̼��������Һ�����һ����������ӷ���ʽΪ��![]() ��
��
�۰�����ڵ����ӷ���ʽΪ��![]() ��
��![]() �� �����Һ������Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��NH2-����c��NH4CO3-����c��NH4+����
�� �����Һ������Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��NH2-����c��NH4CO3-����c��NH4+����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣺
(1)Ԫ��K����ɫ��Ӧ���Ϻ�ɫ��������ɫ��Ӧ�ķ��䲨��Ϊ_______nm(����)��
A.404.4 B.553.5 C.589.2 D.670.8 E.766.5
(2)��̬Kԭ���У��������ռ������ܲ�ķ�����________��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ________________��
(3)��֪Ԫ��M���������Ca5(PO4)3F��һ��Ԫ�ء�Ԫ��M����̬ԭ�����ʧȥ��1������5��������������(�������ܣ��÷���I1��I5��ʾ)�����ʾ��
I1 | I2 | I3 | I4 | I5 | |
������(kJ/mol) | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 |
Ԫ��M����̬�������ϼ���________�ۣ����̬ԭ�ӵ����Ų�ʽΪ____________
(4)PO43��������ԭ�ӵ��ӻ���ʽΪ__________������Ϊ_______________
(5)Ca3(PO4)3F�зǽ���Ԫ�ص縺���ɴ�С��˳��Ϊ________________
(6)����Ԫ�ػ�������ʱ仯˳����ȷ����__________________
A.��һ�����ܣ�Cl��S��P��Si B.���ۼ��ļ��ԣ�HF��HCI��HBr��HI
C.�����ܣ�NaF��NaCl��NaBr��NaI D.���ȶ��ԣ�MgCO3��CaCO3��SrCO3��BaCO3
(7)CaF2�����ṹ��ͼ��ʾ����CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ_____________����֪Ca2+��F���뾶�ֱ�Ϊa cm��b cm�������ӵ�����ΪNA��MΪĦ�������������ܶ�Ϊ___________________g��cm��3(���ػ���)��