��Ŀ����
����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣺
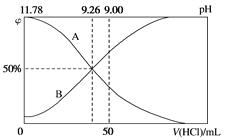
(1)Ԫ��K����ɫ��Ӧ���Ϻ�ɫ��������ɫ��Ӧ�ķ��䲨��Ϊ_______nm(����)��
A.404.4 B.553.5 C.589.2 D.670.8 E.766.5
(2)��̬Kԭ���У��������ռ������ܲ�ķ�����________��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ________________��
(3)��֪Ԫ��M���������Ca5(PO4)3F��һ��Ԫ�ء�Ԫ��M����̬ԭ�����ʧȥ��1������5��������������(�������ܣ��÷���I1��I5��ʾ)�����ʾ��
I1 | I2 | I3 | I4 | I5 | |
������(kJ/mol) | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 |
Ԫ��M����̬�������ϼ���________�ۣ����̬ԭ�ӵ����Ų�ʽΪ____________
(4)PO43��������ԭ�ӵ��ӻ���ʽΪ__________������Ϊ_______________
(5)Ca3(PO4)3F�зǽ���Ԫ�ص縺���ɴ�С��˳��Ϊ________________
(6)����Ԫ�ػ�������ʱ仯˳����ȷ����__________________
A.��һ�����ܣ�Cl��S��P��Si B.���ۼ��ļ��ԣ�HF��HCI��HBr��HI
C.�����ܣ�NaF��NaCl��NaBr��NaI D.���ȶ��ԣ�MgCO3��CaCO3��SrCO3��BaCO3
(7)CaF2�����ṹ��ͼ��ʾ����CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ_____________����֪Ca2+��F���뾶�ֱ�Ϊa cm��b cm�������ӵ�����ΪNA��MΪĦ�������������ܶ�Ϊ___________________g��cm��3(���ػ���)��

���𰸡�A N ���� +2 1s22s22p63s23p64s2��[Ar] 4s2 sp3 109��28�� F>O>P BC 12 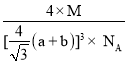
��������
(1)��ɫ��������400nm~430nm��
(2) ��̬Kԭ�ӹ���4�����Ӳ㣬����ܲ�λN���۲�����Ų�ʽΪ4s1��
(3) M�ĵ���������ԶԶ���ڵڶ������ܣ���Ԫ���������2�����ӣ�
(4) ���ݼ۲���ӻ�������ȷ��PO43-�ӻ���ʽ���ռ乹�ͣ�
(5)Ca3(PO4)3F�зǽ���Ԫ��ΪP��O��F��ͬ��������Ԫ��������ҵ縺������ͬ�������϶��µ縺�Լ�С��
(6) A.ͬ����Ԫ�صĵ�һ����������������ƣ��۲���Ӵ��ڳ���������״̬ʱ����һ�����ܴ������Ԫ�صģ�
B. ����ԭ�ӵĵ������ܻ�縺�ԵIJ�ֵԽ����Խ��
C.���Ӱ뾶ԽС������Խ�̣�������Խ��
D.���������ӵİ뾶ԽС����̼���ε����ȶ���Խ����
(7)CaF2�����У��붥��Ca2+����ҵȾ����Ca2+���ھ������ģ�ÿ������Ϊ8���������ã�ÿ������Ϊ2���������ã�ÿ����������Χ��4�������ӣ��ĸ��������γ���������ṹ�������Ӵ��������������ģ�������������������������ķ��������ߴ��ھ����Խ����ϣ��Ҷ��߾�����ھ�����Խ��߳��ȵ�![]() ��
��
(1)��ɫ��������400nm~430nm����ΪA��
(2) ��̬Kԭ�ӹ���4�����Ӳ㣬����ܲ�λN���۲�����Ų�ʽΪ4s1�����ܲ����������Ϊ���Σ�
(3) M�ĵ���������ԶԶ���ڵڶ������ܣ�˵����Ԫ��ʧȥ2������ʱΪ�ȶ��ṹ�����Ԫ���������2�����ӣ���MΪCa��Ԫ��M����̬�������ϼ���+2�����̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p64s2��[Ar] 4s2��
(4) PO43-������ԭ��P�ŵ��Ӷ���=![]() ��a-bx��=
��a-bx��=![]() (5+3-2��4)=0���۲���Ӷ���=4+0=4��Pԭ���ӻ���ʽΪsp3�ӻ������ռ乹��Ϊ�������壬����Ϊ109��28����
(5+3-2��4)=0���۲���Ӷ���=4+0=4��Pԭ���ӻ���ʽΪsp3�ӻ������ռ乹��Ϊ�������壬����Ϊ109��28����
(5)Ca3(PO4)3F�зǽ���Ԫ��ΪP��O��F��ͬ��������Ԫ��������ҵ縺������ͬ�������϶��µ縺�Լ�С���ʵ縺�ԣ�F>O>P��
(6) A.ͬ����Ԫ�صĵ�һ����������������ƣ��۲���Ӵ��ڳ���������״̬ʱ����һ�����ܴ������Ԫ�صģ���Cl��P��S��Si��A����
B. ����ԭ�ӵĵ������ܻ�縺�ԵIJ�ֵԽ����Խ���ۼ��ļ��ԣ�HF��HCl��HBr��HI��B��ȷ��
C.���Ӱ뾶ԽС������Խ�̣�������Խ�����ܣ�NaF��NaCl��NaBr��NaI��C��ȷ��
D.���������ӵİ뾶ԽС����̼���ε����ȶ���Խ���������ȶ��ԣ�BaCO3��SrCO3��CaCO3��MgCO3��D����
��ΪBC��
(7)CaF2�����У��붥��Ca2+����ҵȾ����Ca2+���ھ������ģ�ÿ������Ϊ8���������ã�ÿ������Ϊ2���������ã���CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ��![]() =12��ÿ����������Χ��4�������ӣ��ĸ��������γ���������ṹ�������Ӵ��������������ģ�������������������������ķ��������ߴ��ھ����Խ����ϣ��Ҷ��߾�����ھ�����Խ��߳��ȵ�
=12��ÿ����������Χ��4�������ӣ��ĸ��������γ���������ṹ�������Ӵ��������������ģ�������������������������ķ��������ߴ��ھ����Խ����ϣ��Ҷ��߾�����ھ�����Խ��߳��ȵ�![]() ��Ca2+��F-�뾶�ֱ�Ϊa cm��b cm������Խ��߳���Ϊ4��a+b��cm���ʾ����ⳤ=
��Ca2+��F-�뾶�ֱ�Ϊa cm��b cm������Խ��߳���Ϊ4��a+b��cm���ʾ����ⳤ=![]() (a+b)cm��������Ca2+��Ŀ=8��
(a+b)cm��������Ca2+��Ŀ=8��![]() +6��
+6��![]() =4��F-��ĿΪ8���ʾ�������=4��Mg�������ܶ�=
=4��F-��ĿΪ8���ʾ�������=4��Mg�������ܶ�= gcm-3��
gcm-3��