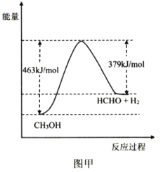
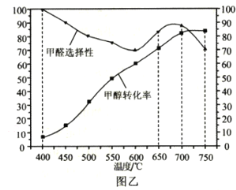
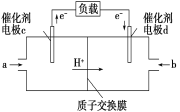
��Ŀ����
����Ŀ������ͼ��ʾװ�ý���ͭ��Ũ����ķ�Ӧ����̽�����������(�г�װ������ȥ)��

(1)�Թܢ��з�����Ӧ�Ļ�ѧ����ʽ��_________���˷�Ӧ��Ũ������_______�ԣ�
(2)���Թܢ��е��Լ�ΪƷ����Һ��ʵ���й۲쵽��������_____________��
(3)���Թܢ��е��Լ�Ϊ���� KMnO4��Һ��ʵ��ʱ���۲쵽��Һ��ɫ����˵�����ɵ��������_____�ԣ�
(4)���Թ��Тڵ��Լ�������ˮ��Һ��д����Ӧ����ʽ__________________��
(5)�Թܢ��е��Լ�Ϊ_____����������______����Ӧ�����ӷ���ʽ��_________��
���𰸡�Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O ǿ�����Ժ����� Ʒ����Һ��ɫ ��ԭ 2H2S+SO2=3S��+2H2O NaOH ��Һ ����β�� SO2+2OH- =
CuSO4+SO2��+2H2O ǿ�����Ժ����� Ʒ����Һ��ɫ ��ԭ 2H2S+SO2=3S��+2H2O NaOH ��Һ ����β�� SO2+2OH- =![]() +H2O
+H2O
��������
���������£�Cu��H2SO4(Ũ) ��Ӧ����CuSO4��SO2��H2O�����ݶ�����������ʽ��
(1).���������£�Cu��H2SO4(Ũ) ��Ӧ����CuSO4��SO2��H2O����Ӧ����ʽΪCu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O��2mol H2SO4���뷴Ӧʱ��1 molH2SO4���ϼ�û�䣬1mol H2SO4��S���ϼ���+6��Ϊ+4�����Ը÷�Ӧ����Ũ����������Ժ�ǿ�����ԣ��ʴ�Ϊ:��Cu+2H2SO4(Ũ��
CuSO4+SO2��+2H2O��2mol H2SO4���뷴Ӧʱ��1 molH2SO4���ϼ�û�䣬1mol H2SO4��S���ϼ���+6��Ϊ+4�����Ը÷�Ӧ����Ũ����������Ժ�ǿ�����ԣ��ʴ�Ϊ:��Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O�����Ժ�ǿ�����ԣ�
CuSO4+SO2��+2H2O�����Ժ�ǿ�����ԣ�
(2).���������ܺ���ɫ���ʷ�Ӧ������ɫ���ʶ�����Ư���ԣ�����������Ư��Ʒ����Һ��ʹƷ����Һ��ɫ�����Կ�������������Һ��ɫ��ȥ���ʴ�Ϊ��Ʒ����Һ��ɫ��
(3).����KMnO4��Һ����ǿ�����ԣ�������������ʹ����ɫ��˵������������л�ԭ�ԣ��ʴ�Ϊ����ԭ��
(4).����������������ԣ�������л�ԭ�ԣ����Զ�������������ˮ��Һ��Ӧ�ķ���ʽΪ��2H2S+SO2=3S��+2H2O���ʴ�Ϊ��2H2S+SO2=3S��+2H2O��
(5).��������Ϊ�������������������꣬����ֱ���ŷţ���������������Һ����β�����գ����������ܺ��������Ʒ�Ӧ���������������ƺ�ˮ�������ӷ�Ӧ����ʽΪ��SO2+2OH-=![]() +H2O���ʴ�Ϊ��NaOH��Һ��β�����գ�SO2+2OH-=
+H2O���ʴ�Ϊ��NaOH��Һ��β�����գ�SO2+2OH-=![]() +H2O��
+H2O��