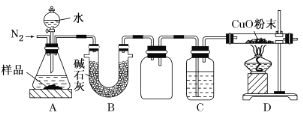
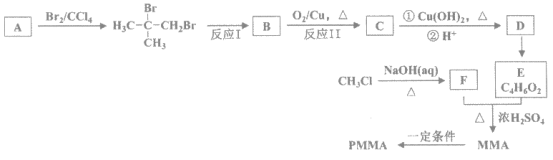
��Ŀ����
����Ŀ����ȩ(HCHO)�׳���ȩ���ڻ�����ҽҩ��ũҩ�ȷ����й㷺��Ӧ�á�
I����ȩ���Ʊ�
��ҵ�����ü״����ⷨ�Ʊ���ȩ����֪��CH3OH(g)![]() HCHO(g)+H2(g) ��H
HCHO(g)+H2(g) ��H
��1���÷�Ӧ�������仯��ͼ����ʾ����H=___kJmol-1��
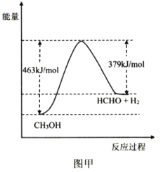
��2��Ϊ���CH3OHת���ʣ���ȡ�Ĵ�ʩ��___��___�����º��������£��÷�Ӧ�ﵽƽ��״̬�ı�־��___�����ţ���
a.���������ܶȱ��ֲ���
b.����������ѹǿ���ֲ���
c.v(CH3OH)����=v(H2)����
d.��ȩ��Ũ�ȱ��ֲ���
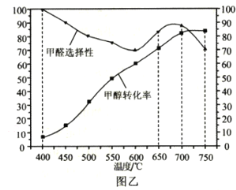
��3��ѡ��Ag/SiO2��ZnO����������400��750��������л������ۣ�ͼ�Ҹ����˼״�ת�������ȩѡ���ԣ�ѡ����Խ��ʾ���ɸ�����Խ�ࣩ�淴Ӧ�¶ȵı仯���ߡ��Ʊ���ȩ����ѷ�Ӧ�¶�Ϊ___�����ţ���������___��
a.400�� b.650�� c.700�� d.750��
��4��T��ʱ����2L�����ܱ������г���1mo1�״���������Ӧ��
��CH3OH(g)![]() HCHO(g)+H2(g)
HCHO(g)+H2(g)
��CH3OH(g)![]() CO(g)+2H2(g)
CO(g)+2H2(g)
ƽ��ʱ�״�Ϊ0.2mol����ȩΪ0.7mo1����Ӧi��ƽ�ⳣ��K=___��
II.��ȩ����;
��5������ȩˮ��Һ��������(NiSO4)��Һ��ϣ������ڻ�ѧ��������Ӧ��������CO2��������÷�Ӧ�����ӷ���ʽΪ___�����ռ���112mLCO2����״������������ת�Ƶ���___ mo1��
���𰸡�+84 �����¶� ����ѹǿ bd c ���¶��¼�ȩ��ѡ���Ժͼ״���ת���ʾ��ϸ� 1.575 HCHO+2Ni2++H2O=2Ni+CO2��+4H+ 0.02
��������
��1��������ͷ�Ӧ��֮���������Ϊ![]() ��
��
��2���÷�Ӧ��������������ӵġ����ȵĿ��淴Ӧ����˸�����������ԭ�������ǿ��Բ��������¶ȡ�����ѹǿ�ķ������ٽ�ƽ�������ƶ������ת���ʣ�������ƽ��ı�־��
a.��Ӧ��������ﶼ�����壬���������ܶ��Ǻ㶨����ģ�a�����
b.�÷�Ӧǰ��������������ȣ���˵�ѹǿ���ֲ���ʱ��˵����Ӧ�Ѵﵽƽ��״̬��b����ȷ��
c.�״��������Ļ�ѧ��������ͬ��������ۺ�ʱ����v(CH3OH)����=v(H2)������c�����
d.����ȩ��Ũ�ȱ��ֲ��䣬˵�����������ʺ�����������ͬ������ʱ�ﵽ��ƽ��״̬��d����ȷ��
��ѡbd��
��3����700��ʱ����ȩѡ���Ժͼ״�ת���ʾ��ϸߣ����700��������ʵ��¶ȣ���ѡc��
��4��ƽ��ʱ�״�Ϊ0.2mol�������0.8mol�״������ģ����м�ȩ��0.7mol��˵����0.7mol�״������˷�Ӧ�٣���0.1mol�״������˷�Ӧ�ڣ��������һ����![]() ������ƽ�ⳣ���ı���ʽ��
������ƽ�ⳣ���ı���ʽ��![]() ��
��
��5��Ȼ�Ƕ�������������ԭΪ���ʣ�����ȩ������Ϊ������̼����˷�Ӧ�����ӷ���ʽΪ![]() ����ȩ�е�̼����0�۴�����������̼�е�̼Ϊ+4�ۣ����ÿ����1��������̼������Ҫת��4�����ӣ���112mL������̼�����ʵ���Ϊ
����ȩ�е�̼����0�۴�����������̼�е�̼Ϊ+4�ۣ����ÿ����1��������̼������Ҫת��4�����ӣ���112mL������̼�����ʵ���Ϊ![]() �����һ��Ҫת��0.02mol���ӡ�
�����һ��Ҫת��0.02mol���ӡ�

����Ŀ���Լ���� CO2 �ĸ�Ч���ò����ܻ��������ů�����Ҷ�����ݽߵ�ʯ����ԴҲ��һ���IJ������ã������������ CO2 ������Ӧ�У�
��Ӧ(i)��2CH4(g)��O2(g)2CO(g)��4H2(g) �� H=��71.4kJmol-1
��Ӧ(ii)��CH4(g)��CO2(g)2CO(g)��2H2(g) �� H=+247.0 kJmol-1
(1)д����ʾ CO ȼ���ȵ��Ȼ�ѧ����ʽ��_____��
(2)�����������Ϊ 2L �ĺ����ܱ������У���ʼʱ��������Ӧ�����������ʣ�����ͬ�¶��½��з�Ӧ(ii)��CH4(g)��CO2(g)2CO(g)��2H2(g) (������������Ӧ)��CO2��ƽ��ת���������ʾ��
���� | ��ʼ���ʵ���(n) / mol | CO2��ƽ��ת���� | |||
CH4 | CO2 | CO | H2 | ||
�� | 0.1 | 0.1 | 0 | 0 | 50�� |
�� | 0.1 | 0.1 | 0.2 | 0.2 | / |
������������˵����Ӧ�ﵽƽ��״̬����_____��
A.v��(CH4) =2v��(CO)
B.�����ڸ����ʵ�Ũ������c(CH4)��c(CO2)=c2(CO)��c2(H2)
C.�����ڻ���������ѹǿ���ٱ仯
D.�����ڻ�������ܶȱ��ֲ���
�ڴﵽƽ��ʱ���������� CO �����ʵ����Ĺ�ϵ���㣺2n(CO)��_____n(CO)��(������������������������)
(3)��һ�����ļ�������������ɷ�Ӧ(i)������������ͬ���ڼס������ֲ�ͬ���������£� ��ͬʱ���ڲ�� CH4 ת�������¶ȱ仯��ϵ��ͼ��ʾ��c ��_____(��������������һ��������һ��δ��)�ﵽƽ��״̬��������_____��

(4)CO2 Ҳ��ͨ��������ϳ��Ҵ����䷴Ӧԭ��Ϊ��2CO2(g)+6H2(g)C2H5OH(g)+3H2O(g) H<0���� m Ϊ��ʼʱ��Ͷ�ϱȣ��� m= n(H2)/ n(CO2)��ͨ��ʵ��õ�����ͼ��
ͼ1 ͼ2
ͼ2 ͼ3
ͼ3
��ͼ 1 ��Ͷ�ϱ���ͬ���¶ȴӸߵ��͵�˳��Ϊ_________��
��ͼ 2 �� m1��m2��m3 �Ӵ�С��˳��Ϊ_________��
��ͼ 3 ��ʾ����ѹΪ 5 MPa �ĺ�ѹ�����£��� m=3 ʱ��ƽ��״̬ʱ�����ʵ����ʵ����������¶ȵĹ�ϵ��T4 �¶�ʱ���÷�Ӧѹǿƽ�ⳣ��KP�ļ���ʽΪ_________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ����������������ݣ����ü���)��
����Ŀ����������ʵ���ʵ��������������ý�����ȷ����
ʵ���ʵ����� | ���� | ʵ����� | |
A | �ô���ʯ�����ᷴӦ��ȡCO2���壬����ͨ��һ��Ũ�ȵ�Na2SiO3��Һ�� | ���ְ�ɫ���� | H2CO3�����Ա�H2SiO3������ǿ |
B | ��ij��Һ�ȵμ������ữ���ٵμ�BaCl2��Һ | ���ְ�ɫ���� | ԭ��Һ�к���SO42-��SO32-��HSO3-�е�һ�ֻ��� |
C | ����ZnƬ�봿CuƬ�õ������ӣ����뵽ϡ������Һ�� | CuƬ��������������� | �����ԣ�Zn��Cu |
D |
| ��������Ϊ��ɫ���ұ������Ϊ��ɫ | �����ԣ�Cl2��Br2��I2 |
A. A B. B C. C D. D
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr�������Ĵ��������������£�

��֪���������ȡҺ�еĽ���������Ҫ��Cr3���������Fe3����Al3����Ca2����Mg2����
��Cr2O72-+H2O2CrO42-+2H+
�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
������ȫʱ��pH | 3.7 | 11.1 | 5.4��>8�ܽ⣩ | 9��>9���ܽ� |
��1��ʵ������18.4mol��L-1��Ũ��������480mL2mol��L-1�����ᣬ����ʱ���ò����������ձ����������ͽ�ͷ�ι��⣬����_______��
��2��H2O2�������ǽ���Һ���е�Cr3��ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��_______��
��3������NaOH��Һʹ��Һ�ʼ��ԣ��ȿ��Գ�ȥijЩ�������ӣ�ͬʱ�ֿ��Խ�Cr2O72��ת��Ϊ______�������Ļ�ѧʽ����
��4�������ӽ�����֬�ķ�Ӧԭ��ΪMn����nNaR=MRn��nNa���������������ӽ�����֬�ɳ�ȥ��Һ���еĽ�����������_____��
��5��д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ��_____��
��6�������ζ����Dzⶨ����Ũ�ȵķ���֮һ��Ϊ�˲ⶨij��ˮ��SCN-��Ũ�ȣ�����0.1000mol��L��1AgNO3����Һ�ζ�����Һ����֪��

���ζ�ʱ��ѡΪ�ζ�ָʾ������_______�����ţ����ζ��յ��������_______��
A��NaCl����B��K2CrO4������C��KI������D��NaCN
��ȡij��ˮ25.00ml,�ζ��յ�ʱ����AgNO3����Һ10.00ml,���ˮ��SCN�������ʵ���Ũ��Ϊ_______��