��Ŀ����
����Ŀ���������������������л��������빦�ܹ�ϵͼ����ͼ1�����ͼ�ش�

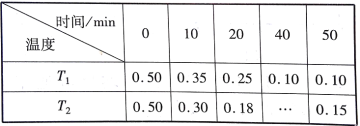
��1��С������ϸ���У�����A��______________������E��______________�����̲����е�H����ˮ�����Եõ�________�ֲ��
��2����ͬ������E��F���������ֽ⣬�������϶����___________��
��3����a��C�������b���������ij������G��������G���ٺ�����ԭ�ӵĸ�����________����G��һ����121�������ṹ�ɵ���״�ṹ�����к�5���ʰ��ᣨ��R��Ϊ-H�����ֱ�λ��26��71��72��99��121λ������ͼ����
![]()
��øE1ר��ˮ��ʰ����Ȼ��˵��ļ�����øE2ר��ˮ��ʰ��ᰱ���˵��ļ�����øE1��ȫ���ú�����Ķ����У�������________���Ȼ���
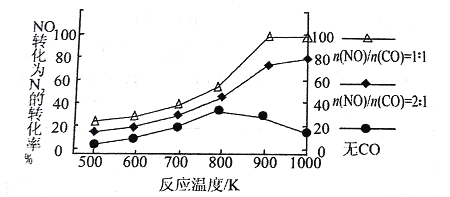
��4��ͼ����ʾС�������ⶨ����������Ҫ���ʵı仯ͼ�����ͼ�ش����⣺
��С�������������Ҫ���л�Ӫ��������________���������Ի�ԭ�ǵ��Լ���_________�������Ի�ԭ�ǵĶ��ٿ���ͨ��________________���жϡ�
�����ӳ���ʱ�����۵��γ���һ�����ữø�Ļ��������й�ϵ��Ϊ����֤���ữø�Ƿ�Ϊ�����ʣ�ʵ�������ʵ�����Թ��м���2ml________���������Թ��м���_______��Ȼ������֧�Թ��зֱ�������˫�����Լ������ʵ�����Թ���ͬ������____________������֤�����ữø�ǵ����ʡ�
���𰸡������� ���� 6 F a+b 4 ���� ����Լ� ש��ɫ����dz ���ữø��Һ �����ĵ�����Һ ��ɫ
��������
����ͼһ��֪��E�Ƕ��ǣ�A�������ǣ�F��֬����B�Ǹ��ͺ�֬���G�ǵ����ʣ�C�ǰ����H�Ǻ��ᣬD�Ǻ��������ͼ����֪��С�����ӳ�������У����Ǻͻ�ԭ�ǵĺ������ͣ����۵ĺ��������ߣ������ʵĺ����������䣬���ӳ�����ۺ�����ߣ�ռ���ص�80%���ҡ�
��1����С������ϸ���У�����A�������ǣ�����E�ǵ��ۡ����̲�����RNA������RNA�Ļ�����ɳɷ��Ǻ��ǡ������A��G��C��U���ּ������˳���ˮ��IJ����Ǻ��ǡ������A��G��C��U���ּ������6�ֲ��
��2����������ȣ�֬���к��н϶��H�������ֽ�ʱ���ĵ������࣬�ͷŵ������ࣻE���ǣ�F��֬��������ͬ������E��F���������ֽ⣬�������϶����F��
��3��a���������γ�b��������ȥ��ˮ�������ǣ�a-b�����������G���ٺ�����ԭ�ӵĸ�����2a-��a-b��=a+b������G��һ����121�������ṹ�ɵ���״�ṹ�����к�5���ʰ��ᣨ��R��Ϊ-H�����ֱ�λ��26��71��72��99��121λ����øE1ר��ˮ��ʰ����Ȼ��˵��ļ�����øE2ר��ˮ��ʰ��ᰱ���˵��ļ�����øE1��ȫ���ú�����Ķ��������1-26��27-71��73-99��100-121����4�����������ٺ���4���Ȼ���
��4���ٷ���ͼ����֪��С�������������Ҫ���л�Ӫ�������ǵ��ۣ���ԭ�dz��õ��Լ�������Լ��������Ի�ԭ�ǵĶ��ٿ���ͨ��ש��ɫ����dz�̶����жϡ�
�ڷ��������֪����ʵ���Ŀ������֤���ữø�Ƿ�Ϊ�����ʣ���ʵ���ԭ���ǵ�������˫�����Լ�������ɫ��Ӧ������ʵ����ƵĶ���ԭ��͵�һ������ԭ��ʵ����Ƶ�˼·�ǣ�ʵ�������ʵ�����Թ��м���2mL���ữø��Һ���������Թ��м�������ĵ�����Һ��Ȼ����������˫�����Լ����۲���ֵ���ɫ��Ӧ�����ʵ�����Թ���ͬ��������ɫ����֤�����ữø�ǵ����ʡ�

����Ŀ��25��ʱ��Ũ�Ⱦ�Ϊ0.1mol��L����Һ����pH���±���ʾ���й�˵����ȷ����
��� | �� | �� | �� | �� |
��Һ |
|
|
|
|
pH | 7.0 | 7.0 | 8.1 | 8.9 |
A. ����ǿ���� ![]()
B. ���ӵ���Ũ�ȣ���>��
C. ���� ![]()
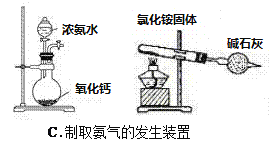
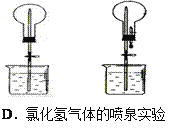
D. �ں͢���![]() ���
���