��Ŀ����
����Ŀ�������ü�������NO2��Ⱦ�����о�����Ӧԭ��ΪCH4+2NO2![]() N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50 molCH4��1.2 mol NO2�����n(CH4)(��λ��mol)��ʱ��仯���й�ʵ�����������ʾ��
N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50 molCH4��1.2 mol NO2�����n(CH4)(��λ��mol)��ʱ��仯���й�ʵ�����������ʾ��
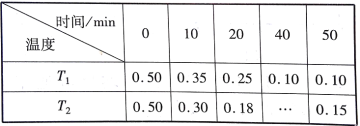
����˵����ȷ���ǣ� ��
A. T1�¶��£�0~20 min�ڣ�NO2�Ľ�������Ϊ0.0125 mol��L-1��min-1
B. ��ʵ�����ݿ�֪ʵ����Ƶ��¶�T1<T2
C. 40minʱ��������T2Ӧ�������Ϊ0.18
D. 0~10 min�ڣ�CH4�Ľ�������T1> T2
���𰸡�B
��������
���ݻ�ѧ��Ӧ���ʵļ��㹫ʽ���¶ȶԷ�Ӧ���ʺͻ�ѧƽ���Ӱ����з�����
A� T1�¶�0~20 min�ڣ�v(NO2)=2v(CH4)=2��(0.50-0.25)mol/(1L��20min)=0.025mol/(L��min)��A�����
B���0~20 min�ڣ�T1�¶�n(CH4)��С��T2�¶�n(CH4)��С��������T1ʱ��Ӧ�����¶Ƚϵ͡�B����ȷ��
C��¶�T1��40minʱ����Ӧ�Ѵﻯѧƽ�⡣�¶�T2��T1����ѧ��Ӧ���죬��40min֮ǰ�ﵽ��ѧƽ�⣬��40minʱ���Ժ��ǻ�ѧƽ�⣬Ӧ�������Ϊ0.15��C�����
D� 0~10 min�ڣ�T1ʱCH4����0.15mol��T2ʱCH4����0.20mol����CH4��������T1< T2��D�����
����ѡB��