��Ŀ����
����Ŀ�����������Ƽ�ȩ(NaHSO2��HCHO��2H2O��M=154.0g��mol-1���׳Ƶ��飬������ˮ�������Ҵ�����120�������ֽ⡣��Na2SO3��SO2��HCHO��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽�����£�
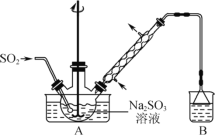
����1����������ƿ�м���һ����Na2SO3��ˮ�������ܽ⣬����ͨ��SO2������ҺpH ԼΪ4���Ƶ�NaHSO3��Һ��
����2����������ƿ�м����Թ�����п�ۺ�һ������ȩ��Һ����80��~90���£���ӦԼ3h����ȴ�����£����ˡ�
����3������Һ���ᾧ�����ˡ�ϴ�ӵȲ������Ƶôֲ�Ʒ��
��1������2�м���п�۷�Ӧʱ����Zn(OH)2���ɣ�д��������Ӧ�Ļ�ѧ����ʽ_____________��Ϊ��ֹ���ɵ�Zn(OH)2������п�۱�����ֹ��Ӧ���У��ɲ�ȡ�Ĵ�ʩ��_____________��
��2������˵���в���ȷ����_____________��
A��װ��B������������β��
B����ʵ��ļ��ȷ�ʽ���ѡ��ˮԡ����
C������2���˵������ɷ�ֻ��Zn(OH)2����
D������3�õ��IJ�Ʒ�����ں����и��º��
��3����ͼװ�ã�����һϵ�в�����ɲ���3�еĽᾧ��ϴ�Ӳ�������ѡ����ʵ���ĸ������ȷ�IJ���˳������(ϴ�Ӳ���ֻ�迼��һ��)��_____����Һת��������©��������������c��f��________��_________��_________��________��d���س����á�_____________
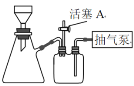
a������Һ�����������������
b������Һ���Ũ������ȴ�ᾧ
c���رջ���A
d������A
e�����Ҵ�ϴ��
f��ȷ�ϳ��
��4����0.5000g��Ʒ��ּ��ȷֽ⣬�ͷų���HCHO��36.00mL0.1000mol��L��1KMnO4��Һ����(������SO2��Ӱ�죬5HCHO+4![]() +12H+=5CO2��+4Mn2++11H2O)������0.1000mol��L��1H2C2O4��Һ�ζ�KMnO4��Һ���ظ�ʵ��3�Σ�ƽ������H2C2O4��Һ�����Ϊ30.00mL������Ʒ�Ĵ���Ϊ_____________��
+12H+=5CO2��+4Mn2++11H2O)������0.1000mol��L��1H2C2O4��Һ�ζ�KMnO4��Һ���ظ�ʵ��3�Σ�ƽ������H2C2O4��Һ�����Ϊ30.00mL������Ʒ�Ĵ���Ϊ_____________��
���𰸡�NaHSO3��Zn��HCHO+3H2O=NaHSO2��HCHO��2H2O+Zn(OH)2 ���� CD b��d��e��c��f 92.4%
��������
��ʼSO2��Na2SO3��Һ��Ӧ����NaHSO3��Ȼ��NaHSO3��Һ��Zn��һ������ȩ��Һ������Ӧ����NaHSO2��HCHO��2H2O��S�Ļ��ϼ۽��ͣ�Zn�Ļ��ϼ����ߣ���������ȴ�ᾧ��
(1)�����Ƶõ���Һ�к���NaHSO3������Zn�ͼ�ȩ���Ƶ�NaHSO2��HCHO��2H2O������S�Ļ��ϼ�Ϊ��2����S�Ļ��ϼ۴�NaHSO3�еģ�4���͵���2��Zn�Ļ��ϼ۴�0���ߵ���2�����ϼ�������ȣ���NaHSO3��Zn��ϵ����Ϊ1��1���ٸ��������غ���ƽ����ѧ����ʽΪNaHSO3��Zn��HCHO+3H2O=NaHSO2��HCHO��2H2O+Zn(OH)2��ͨ�����裬����ʹZn(OH)2����Zn�ı��渻����
(2)A����Ӧ�����У�û�з�Ӧ��SO2�ݳ������װ��B�����þ�������β������ֹ��Ⱦ�ջ�����A��ȷ����ѡ��
B����Ӧ�¶���80��90�棬��ˮԡ���ȣ����ԽϺõؿ����¶ȣ��Ҽ��ȸ����ȣ�B��ȷ����ѡ��
C�����˵���������Zn(OH)2�����й�����Zn��C���������⣻
D����Ʒ����120�������ֽ⣬���ܸ��º�ɣ�D���������⣻
��ѡCD��
(3)�õ���Һ��Ӧ�������������Ũ�������Է�ֹ�¶ȹ���ʹ����ֽ⣬Ҳ���Է�ֹ����������������ת�ƹ�Һ���������ã��رջ���A��ȷ�ϳ�ɣ���ϴ�ӳ�����ϴ�ӳ���ǰ��Ҫ����A���ټ���ϴ�Ӽ����ڹرջ���A��ȷ�ϳ�ɣ������������˳��Ϊ��b����Һת��������©��������������c��f��d��e��c��f��d���س����ã�
(4)KMnO4������HCHO��H2C2O4�����ܵ�KMnO4��ȥ����H2C2O4��KMnO4�͵õ�����HCHO��KMnO4���Ӷ�����õ���Ʒ�Ĵ��ȡ��ܵ�KMnO4�����ʵ���n1=36mL��10��3mL��L��1��0.1mol=3.6��10��3mol��KMnO4��H2C2O4�Ļ�ѧ����ʽΪ5H2C2O4��2KMnO4��3H2SO4=K2SO4��2MnSO4��10CO2����8H2O�����ĵ�n(H2C2O4)=30mL��10��3mL��L��1��0.1mol��L��1=3��10��3mol��������H2C2O4��KMnO4�����ʵ���n2=![]() ������HCHO��KMnO4�����ʵ���n=n1-n2=3.6��10��3mol-1.2��10��3mol=2.4��10��3mol������Ʒ��HCHO�����ʵ���n(HCHO)=
������HCHO��KMnO4�����ʵ���n=n1-n2=3.6��10��3mol-1.2��10��3mol=2.4��10��3mol������Ʒ��HCHO�����ʵ���n(HCHO)=![]() ����Ʒ��NaHSO2��HCHO��2H2O�����ʵ���Ϊ3��10��3mol������Ʒ������m=154.0g��mol-1��3��10��3mol=0.462g������Ʒ�Ĵ���=
����Ʒ��NaHSO2��HCHO��2H2O�����ʵ���Ϊ3��10��3mol������Ʒ������m=154.0g��mol-1��3��10��3mol=0.462g������Ʒ�Ĵ���=![]() ��
��