��Ŀ����
����Ŀ�����ᶡ�����������������
���� | 1-���� | ���ᶡ�� | |
�۵�(��) | 16.6 | ��89.5 | ��73.5 |
�е�(��) | 117.9 | 117 | 126.3 |
�ܶ�(g/cm3) | 1.05 | 0.81 | 0.88 |
ˮ���� | ���� | ����(9g/100gˮ) | �� |
ʵ��������ͼ��ʾ��ʵ��װ����ȡ���ᶡ����
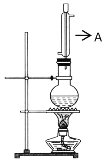
(1)����A������___��
(2)д����ȡ���ᶡ���Ļ�ѧ����ʽ��__��
(3)��ʵ����������������������ᶡ���⣬���������ɵ��л���������__(д��һ�ֽṹ��ʽ)��
(4)������Ӧ��һ�����淴Ӧ��Ϊ���1-������ת���ʣ��ɲ�ȡ�Ĵ�ʩ��__(д������)��
(5)���Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У���Ҫ����__(��𰸱��)��
(6)��60g������37g1-������Ӧ��ʵ���еõ����ᶡ��������Ϊ40.6g�������ᶡ���IJ���Ϊ___��
���𰸡�ֱ�������� CH3COOH��HOCH2CH2CH2CH3![]() CH3COOCH2CH2CH2CH3��H2O CH3CH2CH2CH2OCH2CH2CH2CH3��CH3CH2CH=CH2 ����������������С������Ũ��(������������) AB 70%
CH3COOCH2CH2CH2CH3��H2O CH3CH2CH2CH2OCH2CH2CH2CH3��CH3CH2CH=CH2 ����������������С������Ũ��(������������) AB 70%
��������
�����1-������Ũ������������·���������Ӧ�������ᶡ�����Ʊ����ᶡ�������У���Ũ���������£�1-�����ܹ�������ȥ��Ӧ����1-��ϩ���ܹ�������������ˮ���������ѣ�����������������Ӧ�ǿ��淴Ӧ������Ӱ�컯ѧƽ������ؽ��н�����1-����ת���ʣ�����������һ�ַ�Ӧ��Ũ�Ȼ�����������Ũ�ȣ��ᴿ���������ᶡ��ʱ����Ҫͨ����Һ���������ᶡ���IJ���=![]() ��100%���ݴ˷������
��100%���ݴ˷������
(1)����A������ֱ�������ܣ�
(2)�����1-������Ũ������������·���������Ӧ�������ᶡ������ȡ���ᶡ���Ļ�ѧ����ʽ��CH3COOH��HOCH2CH2CH2CH3![]() CH3COOCH2CH2CH2CH3��H2O��
CH3COOCH2CH2CH2CH3��H2O��
(3)���ݷ������Ʊ����ᶡ�������У���Ũ���������£�1-�����ܹ�������ȥ��Ӧ����1-��ϩ���ܹ�������������ˮ���������ѣ����л���������ܵĽṹ��ʽΪCH3CH2CH2CH2OCH2CH2CH2CH3��CH3CH2CH=CH2��
(4)������Ӧ��һ�����淴Ӧ���ӻ�ѧƽ���ƶ��ĽǶȷ�����֪�����������������С������Ũ��(������������)����ʹƽ�����ƣ����������1-�����������ʣ�
(5)���Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�������뾭����ȡ���Һ����ѡAB��
(6)60g��������ʵ���Ϊ![]() =1mol��37g1-���������ʵ���Ϊ
=1mol��37g1-���������ʵ���Ϊ![]() =0.5mol�����ݷ�ӦCH3COOH��HOCH2CH2CH2CH3
=0.5mol�����ݷ�ӦCH3COOH��HOCH2CH2CH2CH3![]() CH3COOCH2CH2CH2CH3��H2O��֪�������������1-�����������㣬0.5mol��1-������ȫ��Ӧʱ�������ᶡ�������ʵ���Ϊ0.5mol���������������ᶡ��������Ϊ0.5mol��116g/mol=58g��ʵ���еõ����ᶡ��������Ϊ40.6g�������ᶡ���IJ���=
CH3COOCH2CH2CH2CH3��H2O��֪�������������1-�����������㣬0.5mol��1-������ȫ��Ӧʱ�������ᶡ�������ʵ���Ϊ0.5mol���������������ᶡ��������Ϊ0.5mol��116g/mol=58g��ʵ���еõ����ᶡ��������Ϊ40.6g�������ᶡ���IJ���=![]() ��100%=
��100%=![]() ��100%=70%��
��100%=70%��

����Ŀ���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ��Ԫ�ء�
A | R | |||||||||||||||||
B | C | D | E | F | T | |||||||||||||
G | H | I | J | K | L | |||||||||||||
M | N | O | ||||||||||||||||
�Իش���������(ע�⣺ÿ���е���ĸ����Ϊ�ϱ��е���ĸ���ţ�����ΪԪ�ط���)
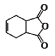
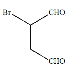
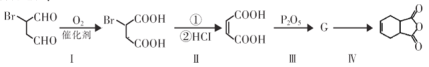
(1)N�ĵ��ʺ�ˮ������Ӧ�����ɹ���X����I�ĵ�����X�����·�Ӧ�Ļ�ѧ����ʽ______��
(2)D�ļ���̬�⻯���VSEPRģ�͵�����Ϊ______��
(3)��A��C��D�γɵ�ACD�����У���������������=______��
(4)Ԫ��M�Ļ�����(ME2L2)���л��ϳ��п������������Ȼ��������������л��ﷴӦ���ش����⣺
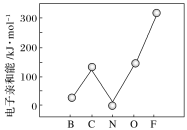
��ME2L2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ��ݴ˿��ж�ME2L2��______(���������������Ǽ�����)���ӡ�
�ڽ�N��O�ĵ����õ������Ӻ����D������������Ӧ��ˮ����Ũ��Һ�У����Ƴ�ԭ��أ�����ɸ������ϵ�Ԫ�ص���Χ���ӹ����ʾʽΪ______��
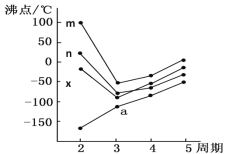
(5)��ͼ�������߷ֱ��ʾ��A�塢��/span>A�塢��A�塢��A��Ԫ����̬�⻯��е�仯����E���⻯�����ڵ�������______(��m��n��x��y)��