��Ŀ����
��Ҫ���������С��.
(1��0.5 mol H2O������Ϊ g������____________������,___________�����ӡ�
(2) 0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ__________________��
��3������50 mL 0.2 mol/L CuSO4��Һ����ҪCuSO4_____________g����ҪCuSO4��5H2O _____g��
��4����ͼ����У��ѧʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�
�ٸ�Ũ��������ʵ���Ũ��_____________��
���ø�Ũ��������200mL1mol/L��ϡ���ᣬ��Ͳ������ȡ��Ũ����������_________mL��
(1) 9 0.5NA��3.01x1023 5NA��3.01x1024 (2)108g/mol
(3)1.6 2.5 (4) 18.4mol/L 10.9
���������������1��H2O��Ħ������Ϊ18g/mol����0.5mol H2O������Ϊ0.5mol��19g/mol=9g�����з�������0.5mol��NAmol-1=0.5NA��һ��ˮ��������10�����ӣ���������0.5mol��10��NAmol-1=5NA��
�ʴ�Ϊ��9��0.5NA��3.01��1023��5NA��3.01��1024��
��2������n��M=m������ϵ����õ�Ħ��������M="m/M=1.08g/0.01mol=" =108g/mol���ʴ�Ϊ��108g/mol��
��3����CuSO4����50mL0.2mol?L-1CuSO4��Һ����ҪCuSO4����Ϊ��0.05��0.2mol/L��160g/mol=1.6g����CuSO4?5H2O����50mL0.2mol?L-1CuSO4��Һ����ҪCuSO4?5H2O����Ϊ��0.05��0.2mol/L��250g/mol=2.5g���ʴ�Ϊ��1.6��2.5��
(4)��Һϡ��ǰ�����ʵ����ʵ������䣬����Ũ��������ΪV�����У�0.2L��1mol/L=V��18.4mol/L��V=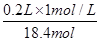 =0.0109L����10.9ml���ʴ�Ϊ��18.4mol/L��10.9��
=0.0109L����10.9ml���ʴ�Ϊ��18.4mol/L��10.9��
���㣺�����ӵ����������ʵ���Ũ�ȣ����ʵ�������ؼ��㣻����һ�����ʵ���Ũ�ȵ���Һ��

��.ʵ��������1mol/L Na2CO3��Һ250ml��
��1����Ҫ����Na2CO3 g����2������Һ�е���������ĿΪ ����
��3����Ҫ���ʵ���Ũ��Ϊ5mol/L ��Na2CO3��Һ ml��
��4��������Һ������ϡ���ᷴӦ�������������ڱ�״���µ����Ϊ L��
��5�����Ƹ���Һ�IJ���˳����(����ĸ��ʾ,���ظ�ʹ��) ��
| A������ | B��ϴ�� | C������ | D���ܽ� E��ҡ�� F��ת�� |
ʵ����Ҫ0.10mol/LNaOH��Һ470mL��������Һ����������ش��������⣺
��1��ʵ���г���������ƽ���ձ�������������Ͳ��ҩ�����Ҫ�����������У� ��
��2�����ݼ����֪������NaOH������Ϊ g��
��3������ʱ��������ƿ����Һ�İ�Һ��������̶������У��Ǻ�ƿ�������һ�������� ��
��4������ʱ,�������ˮ�����̶��ߣ������ȡ�Ĵ�ʩ�ǣ� ��
��5�����в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ�͵��� ����Ӱ����� ��
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |