��Ŀ����
�����Ȼ�ѧ����ʽ����ȷ���ǣ�
| A��4g��������ȫȼ������SO2���ų�37 kJ������S(s)+O2(g)=SO2(g)��H= -296kJ/mol |
| B��1molN2��3molH2��ij�ܱ������з�Ӧ�ų�73kJ��������Ӧ���Ȼ�ѧ����ʽΪ�� N2(g)+3H2(g)  2NH3(g)��H= -73kJ/mol 2NH3(g)��H= -73kJ/mol |
| C������ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g) ==CO2(g)+ 2H2O(g)��H=-890.3kJ��mol-1 |
| D��ǿ��ǿ����к���Ϊ- 57.3 kJ/mol�� |
A
�������������A��4g��������ȫȼ������SO2���ų�37 kJ������1mol��������ȫȼ������SO2���ų�37 ��8kJ������S(s)+O2(g)=SO2(g)��H= -296kJ/mol������ȷ��B��1molN2��3molH2��ij�ܱ������з�Ӧ�ų�73kJ��������Ϊ��Ӧ�ǿ���ģ����ܽ��е��ף��ʷ�Ӧ���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g) 2NH3(g) ��H��-73kJ/mol��ԭ˵������ȷ��C������ı�ȼ����Ϊ-890.3kJ��mol-1��H2OΪҺ̬���ʼ���ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g) ==CO2(g)+ 2H2O(l) ��H=-890.3kJ��mol-1��ԭ˵������ȷ��D��ǿ��ǿ����к���Ϊ- 57.3 kJ/mol����Ba(OH) 2(aq)+H2SO4(aq)=BaSO4(s)+2H2O(l)��BaSO4(s)���ɣ��ʦ�H��-114.6 kJ/mol��ԭ˵������ȷ��
2NH3(g) ��H��-73kJ/mol��ԭ˵������ȷ��C������ı�ȼ����Ϊ-890.3kJ��mol-1��H2OΪҺ̬���ʼ���ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g) ==CO2(g)+ 2H2O(l) ��H=-890.3kJ��mol-1��ԭ˵������ȷ��D��ǿ��ǿ����к���Ϊ- 57.3 kJ/mol����Ba(OH) 2(aq)+H2SO4(aq)=BaSO4(s)+2H2O(l)��BaSO4(s)���ɣ��ʦ�H��-114.6 kJ/mol��ԭ˵������ȷ��
���㣺���ȷ�Ӧ����ʽ����ȷ���жϡ�

��������������ȷ���ǣ� ��
| A��CS2ΪV�εļ��Է��� |
B��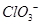 �Ŀռ乹��Ϊƽ�������� �Ŀռ乹��Ϊƽ�������� |
| C��SF6����6����ȫ��ͬ�ijɼ����Ӷ� |
D��SiF4��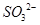 ������ԭ�Ӿ�Ϊsp3�ӻ� ������ԭ�Ӿ�Ϊsp3�ӻ� |
��1��Niԭ�ӵĺ�������Ų�ʽΪ__________________________________��
��2��NiO��FeO�ľ���ṹ���;����Ȼ��Ƶ���ͬ��
 ��
��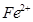 �����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO_________________FeO���������������
�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO_________________FeO�����������������3��NiO������Ni��O����λ���ֱ�Ϊ_________________��_________________��
��4�����������磨La���γɵĺϽ���һ�����õĴ�����ϣ��侧���ṹʾ��ͼ������ͼ��ʾ���úϽ�Ļ�ѧʽΪ_________________��

��5������ͪ뿳����ڼ���
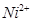 ����ϡ��ˮ�����У�����ͪ���
����ϡ��ˮ�����У�����ͪ���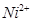 ��Ӧ�������ʺ�ɫ��������ṹ������ͼ��ʾ��
��Ӧ�������ʺ�ɫ��������ṹ������ͼ��ʾ���ٸýṹ�У�̼̼֮��Ĺ��ۼ������ǦҼ���̼��֮��Ĺ��ۼ�������_________________������֮���γɵĻ�ѧ����_________________��
�ڸýṹ�У�����֮������ۼ���ɴ���_________________����
�۸ýṹ�У�̼ԭ�ӵ��ӻ����������_________________��
��֪2H2��g����O2��g��= 2H2O��l�� ��H=��569��6 kJ��mol��1�� 2H2O��g��= 2H2��g����O2��g�� ��H=��482��1 kJ��mol��1������1 gҺ̬H2O������ʱ���յ�������
| A��2��43 kJ | B��4��86 kJ | C��43��8 kJ | D��87��5 kJ |
���б仯���������ȷ�Ӧ����
��Һ̬ˮ���� �ڽ��������ȱ�Ϊ��ɫ��ĩ�� ��Ũ����ϡ�͡�
������طֽ��������� ����ʯ����ˮ��Ӧ������ʯ��
| A���ۢ� | B���ڢ� | C���٢ܢ� | D���ڢ� |
���й��ڻ�ѧ��Ӧ��������˵����ȷ����
| A���кͷ�Ӧ�����ȷ�Ӧ |
| B��ȼ���Ƿ��ȷ�Ӧ |
| C����ѧ�����ѷų����� |
| D����Ӧ����������������������һ����� |
���������������������Ӧ���Ȼ�ѧ����ʽ(25�棬101 kPa)��
��C4H10(g)��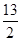 O2(g)===4CO2(g)��5H2O(l) ��H����2 878 kJ��mol��1
O2(g)===4CO2(g)��5H2O(l) ��H����2 878 kJ��mol��1
��C4H10(g)��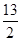 O2(g)===4CO2(g)��5H2O(g) ��H����2 658 kJ��mol��1
O2(g)===4CO2(g)��5H2O(g) ��H����2 658 kJ��mol��1
��C4H10(g)��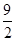 O2(g)===4CO(g)��5H2O(l) ��H����1 746 kJ��mol��1
O2(g)===4CO(g)��5H2O(l) ��H����1 746 kJ��mol��1
��C4H10(g)��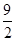 O2(g)===4CO(g)��5H2O(g) ��H����1 526 kJ��mol��1
O2(g)===4CO(g)��5H2O(g) ��H����1 526 kJ��mol��1
�ɴ��жϣ��������ȼ������ (����)
| A����H����2 878 kJ��mol��1 | B����H����2 658 kJ��mol��1 |
| C����H����1 746 kJ��mol��1 | D����H����1 526 kJ��mol��1 |
����˵������ȷ����
| A�������Ҵ���Ӧ���ƿ�����Ҵ�Һ������ |
| B����ȥ���������в��������ᣬ���ñ���Na2CO3��Һϴ�Ӻ��Һ |
| C�����������仯�����ʱ仯���ǻ�ѧ�仯 |
| D����ѧ��Ӧ�ﵽƽ��״̬ʱ��ֻҪ�������ı䣬�����ʵ�Ũ�ȾͲ��ٸı� |
���б仯Ϊ���ȵĻ�ѧ��Ӧ���� 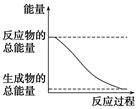
| A��H2O(g)===H2O(l)����H����44��0 kJ��mol��1 |
| B��2HI(g)===H2(g)��I2(g) ��H����14��9 kJ��mol��1 |
| C���γɻ�ѧ��ʱ���ų�862 kJ�����Ļ�ѧ��Ӧ |
| D�������仯����ͼ��ʾ�Ļ�ѧ��Ӧ |