��Ŀ����
���������������������Ӧ���Ȼ�ѧ����ʽ(25�棬101 kPa)��
��C4H10(g)��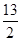 O2(g)===4CO2(g)��5H2O(l) ��H����2 878 kJ��mol��1
O2(g)===4CO2(g)��5H2O(l) ��H����2 878 kJ��mol��1
��C4H10(g)��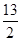 O2(g)===4CO2(g)��5H2O(g) ��H����2 658 kJ��mol��1
O2(g)===4CO2(g)��5H2O(g) ��H����2 658 kJ��mol��1
��C4H10(g)��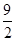 O2(g)===4CO(g)��5H2O(l) ��H����1 746 kJ��mol��1
O2(g)===4CO(g)��5H2O(l) ��H����1 746 kJ��mol��1
��C4H10(g)��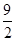 O2(g)===4CO(g)��5H2O(g) ��H����1 526 kJ��mol��1
O2(g)===4CO(g)��5H2O(g) ��H����1 526 kJ��mol��1
�ɴ��жϣ��������ȼ������ (����)
| A����H����2 878 kJ��mol��1 | B����H����2 658 kJ��mol��1 |
| C����H����1 746 kJ��mol��1 | D����H����1 526 kJ��mol��1 |
A
�������������ȼ��������101 kPaʱ��1 mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������������ɵ�������CO2(g)��H2O(l)��Ӧ�÷�Ӧ�ټ��㡣��A��ȷ��
���㣺�����Ȼ�ѧ����ʽ����ȼ���ȵķ�����

��֪���з�Ӧ���Ȼ�ѧ����ʽ��6C(s)+5H2(g)+3N2(g)+9O2(g)=2C3H5(ONO2)3(l) ��H1
2 H2(g)+O2(g)=2H2O(g) ��H2
C(s)+O2(g)=CO2(g) ��H3
��Ӧ4C3H5(ONO2)3(l)=12CO2(g)+10H2O(g)+O2(g)+6N2(g)�ġ�HΪ�� ��
| A��12��H3+5��H2��2��H1 | B��2��H1��5��H2��12��H3 |
| C��12��H3��5��H2��2��H1 | D����H1��5��H2��12��H3 |
��֪25 �桢101 kPa�£����з�Ӧ
C(ʯī) + O2(g) === CO2(g) ��ȼ��1 mol C(ʯī)����393.51 kJ��
C(���ʯ) + O2(g) === CO2(g)��ȼ��1 mol C(���ʯ)����395.41 kJ��
���Եó��Ľ�����
| A�����ʯ��ʯī�ȶ� | B��1 molʯī�����е�������1 mol���ʯ�� |
| C�����ʯת���ʯī�������仯 | D��ʯī�ͽ��ʯ����̼��ͬλ�� |
��101kPa 25��ʱ��1.0g����������ȫȼ������Һ̬ˮʱ�ų�����52.0kJ��������ȼ�յ��Ȼ�ѧ����ʽΪ
A��C2H6(g) �� 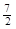 O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1 O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1 |
| B��2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(g)��H =��1560kJ��mol��1 |
| C��2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(l)��H =��3120 kJ��mol��1 |
D��C2H6(g) ��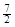 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1 |
�����Ȼ�ѧ����ʽ����ȷ���ǣ�
| A��4g��������ȫȼ������SO2���ų�37 kJ������S(s)+O2(g)=SO2(g)��H= -296kJ/mol |
| B��1molN2��3molH2��ij�ܱ������з�Ӧ�ų�73kJ��������Ӧ���Ȼ�ѧ����ʽΪ�� N2(g)+3H2(g)  2NH3(g)��H= -73kJ/mol 2NH3(g)��H= -73kJ/mol |
| C������ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g) ==CO2(g)+ 2H2O(g)��H=-890.3kJ��mol-1 |
| D��ǿ��ǿ����к���Ϊ- 57.3 kJ/mol�� |
���շ�ӦBr+H2��HBr+H�������淴Ӧ���̱仯��ʾ��ͼ��������������ȷ����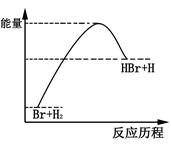
| A������ӦΪ���ȷ�Ӧ |
| B���÷�Ӧ���淴Ӧ�����ȹ��� |
| C��HBr������һ������H2������ |
| D����Ӧ����е�������������������е������� |
�������һ�����ᣬ��������ʴ��������֪25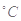 ʱ��
ʱ��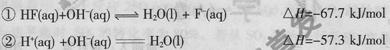
��20Ml0.lmol/L��������еμ�0.lmol/L��NaOH V mL������˵����ȷ����
A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��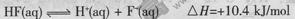 |
B����V="20" mLʱ����Һ�У� |
C����V="20" mLʱ����Һ�У� |
D����v>0ʱ����Һ��һ������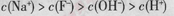 |
�����豸����ʱ������ѧ��ת��Ϊ���ܵ���
| A | B | C | D |
 |  |  |  |
| ��̫���ܵ�� | ����ӵ�� | ̫���ܼ����� | ȼ���� |
��֪��Ӧ�� ��2C(s)��O2(g)��2CO��g����H����221kJ/mol
��H��(aq)��OH��(aq)��H2O��1������H����57.3kJ/mol ���н�����ȷ���ǣ� ��
| A��̼��ȼ���ȴ���110.5kJ/mol |
| B����Ӧ�ٷų�������Ϊ221kJ |
| C��ϡ������ϡ��ˮ��Һ��Ӧ���к���Ϊ��57.3kJ/mol |
| D��ϡ������ϡNaOH��Һ��Ӧ����1molˮ���ų�57.3kJ���� |