��Ŀ����
����ɻ��õ�������������(NH4ClO4)�Ļ����Ϊ����ȼ�ϣ���ȼʱ����������������������立�Ӧ���䷽��ʽ�ɱ�ʾΪ��2NH4ClO4  N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
 N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��| A����Ӧ���ڷֽⷴӦ |
| B��������Ӧ˲������������������ƶ�����ɻ����� |
| C����Ӧ�������仯��˵����Ҫ�ǻ�ѧ��ת��Ϊ���ܺͶ��� |
| D���ڷ�Ӧ�и������ֻ������������ |
D
���������A���������ѧ����ʽ�������÷�Ӧ���ڷֽⷴӦ����ȷ��B���������ѧ����ʽ������������Ӧ˲������������������ƶ�����ɻ����У���ȷ��C����Ӧ�������仯��˵����Ҫ�ǻ�ѧ��ת��Ϊ���ܺͶ��ܣ���ȷ��D���ڷ�Ӧ�и�������е�����Ԫ�ر���������Ԫ�ر���ԭ��������識��������������ǻ�ԭ��������

��ϰ��ϵ�д�
�����Ŀ

 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol ��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�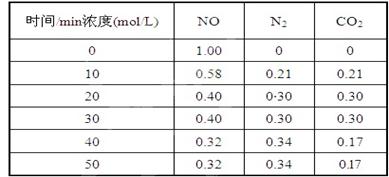



 O2(g) H2O(g) ��H="-241.8" kJ��mol-1 ��
O2(g) H2O(g) ��H="-241.8" kJ��mol-1 �� 2NO(g)����H>0
2NO(g)����H>0 CH3OH(g)
CH3OH(g) 