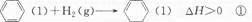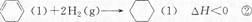��Ŀ����
��7�֣�(1)���������ھ�������������ˮ������������ҵ�������ΪƯ����
�ٳ������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)+O3(g)===3Ag2O(s)����H =" -235.8" kJ/mol;
��֪��2 Ag2O(s)===4Ag(s)+O2(g)����H = +62.2kJ/mol;
��Ӧ 2O3��g��= 3O2��g�� �ġ�H = kJ/mol��
�ڿ�ѧ��P��Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в�
�����������������������ɹ������⣬�����缫��ӦʽΪ ��
 ��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
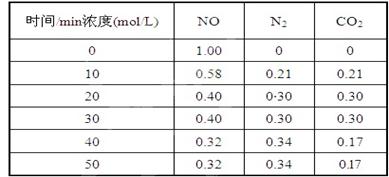
����10 min��20 min��ʱ����ڣ���CO2��ʾ�ķ�Ӧ����Ϊ ��
��д���÷�Ӧ��ƽ�ⳣ���ı���ʽK= ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬���� (�������ĸ)��
��30 minʱ�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� (����������䡱��С��)��
�ٳ������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)+O3(g)===3Ag2O(s)����H =" -235.8" kJ/mol;
��֪��2 Ag2O(s)===4Ag(s)+O2(g)����H = +62.2kJ/mol;
��Ӧ 2O3��g��= 3O2��g�� �ġ�H = kJ/mol��
�ڿ�ѧ��P��Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в�
�����������������������ɹ������⣬�����缫��ӦʽΪ ��
 ��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2(g)+CO2(g) ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��) �����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
����10 min��20 min��ʱ����ڣ���CO2��ʾ�ķ�Ӧ����Ϊ ��
��д���÷�Ӧ��ƽ�ⳣ���ı���ʽK= ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬���� (�������ĸ)��
| A��������ѹǿ���ֲ��� | B��2v��(NO)=v��(N2) |
| C��������CO2������������� | D�����������ܶȱ��ֲ��� |
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� (����������䡱��С��)��
��7�֣�����1����-285kJ/mol��
��3O2+6H++6e-=3H2O2��
��2������1����0.009/mol/��L.min��
��0.56��
��C��D
�ܼ�СCO2Ũ��
�� ���䡣
��3O2+6H++6e-=3H2O2��
��2������1����0.009/mol/��L.min��
��0.56��
��C��D
�ܼ�СCO2Ũ��
�� ���䡣
�����������1���٢�6Ag��s��+O3��g���T3Ag2O��s������H=-235.8kJ?mol-1��
��2Ag2O��s���T4Ag��s��+O2��g������H=+62.2kJ?mol-1�����ݸ�˹���ɿ�֪�١�2+
�ڡ�3�ɵõ���2O3��g��=3O2��g������Ӧ�ȡ�H=��-235.8kJ?mol-1����2+��+62.2kJ?mol-1��
��3=-285kJ/mol��
�����������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в�������缫��ӦʽΪ3H2O-6e-=O3��+6H+�����������������õ������ɹ������⣬��缫��ӦʽΪ3O2+6H++6e-=3H2O2��
��2������10 min��20 min��ʱ����ڣ�CO2��Ũ������0.09mol/L,������CO2��ʾ�ķ�Ӧ����Ϊ0.09mol/L/10min=0.009/mol/��L.min����
�ڷ�Ӧ���е�20minʱ��ƽ��״̬����ʱNO��N2��CO2��ƽ��Ũ�ȷֱ���0.4mol/L��0.3mol/L��
0.3mol/L������ƽ�ⳣ��K=0.32mol/L/0.42mol/L=0.5625��
��A���÷�Ӧ�Ƿ�Ӧǰ������ѹǿ����ķ�Ӧ������ѹǿ���䲻���жϷ�Ӧ�Ƿ��ƽ��״̬������B��ƽ��ʱ��v��(NO)=2v��(N2)������C����Ӧ��ʼʱ������̼���������һֱ����ƽ��״̬ʱ������CO2������������䣬��ȷ��D���÷�Ӧ�й�����룬�������������һֱ��������������䣬����������ܶ�һֱ�ڱ䣬����ƽ��ʱ�������ܶȲ��ٱ仯����ȷ����ѡCD��
�ܴӱ������ݿ�֪��40minʱNO��Ũ�ȼ�С��������Ũ����������̼��Ũ�ȼ�С�������ж�ƽ�������ƶ����ı�������Ǽ�С������̼��Ũ�ȣ�ʹƽ�������ƶ���
�ݸ÷�Ӧ�Ƿ�Ӧǰ����������ʵ�������ķ�Ӧ���ı�NO��Ũ�ȴﵽ��ƽ���ǵ�Чƽ�⣬����NO��ת���ʲ��䡣

��ϰ��ϵ�д�
�����Ŀ
 CO2(g) ��H����437��3 kJ?mol��1
CO2(g) ��H����437��3 kJ?mol��1 CO(g) + H2(g) ��H�� kJ?mol��1������÷�Ӧ��S����133��7 J��K��1��mol��1 �÷�Ӧ�ڳ��£�25 �棩���ܷ��Է����У�����G=��H-T��S�� (��ܡ����ܡ�����д���ж�����)��
CO(g) + H2(g) ��H�� kJ?mol��1������÷�Ӧ��S����133��7 J��K��1��mol��1 �÷�Ӧ�ڳ��£�25 �棩���ܷ��Է����У�����G=��H-T��S�� (��ܡ����ܡ�����д���ж�����)�� 2NH3 ��H="a" kJ��mol-1���Ը��ݱ������м������ݹ���a����ֵΪ ��
2NH3 ��H="a" kJ��mol-1���Ը��ݱ������м������ݹ���a����ֵΪ �� N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� �� FeS ��
FeS ��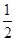 Cl2(g)===NaCl(s)����H1
Cl2(g)===NaCl(s)����H1 HCHO(g)+H2(g),�״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ���ش���������:
HCHO(g)+H2(g),�״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ���ش���������: