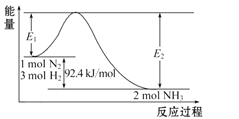
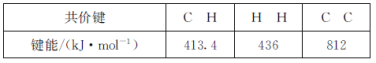
题目内容
【题目】已知反应:
H2(g)+![]() O2(g)
O2(g)![]() H2O(g) △H1
H2O(g) △H1
![]() N2(g)+O2(g)
N2(g)+O2(g)![]() NO2(g) △H2
NO2(g) △H2
![]() N2(g)+
N2(g)+![]() H2(g)
H2(g)![]() NH3(g) △H3
NH3(g) △H3
则反应4NH3(g)+7O2(g)=4NO2(g)+6H2O(g)的△H为( )
A.△H1+△H2+△H3B.2△H1+2△H2-2△H3
C.6△H1+4△H2+4△H3D.6△H1+4△H2-4△H3
【答案】D
【解析】
根据盖斯定律进行计算。
①H2(g)+![]() O2(g)
O2(g)![]() H2O(g) △H1
H2O(g) △H1
②![]() N2(g)+O2(g)
N2(g)+O2(g)![]() NO2(g) △H2
NO2(g) △H2
③![]() N2(g)+
N2(g)+![]() H2(g)
H2(g)![]() NH3(g) △H3
NH3(g) △H3
根据盖斯定律可知,6×①+4×②-4×③可得反应4NH3(g)+7O2(g)=4NO2(g)+6H2O(g) △H=6△H1+4△H2-4△H3;答案选D。

练习册系列答案
相关题目