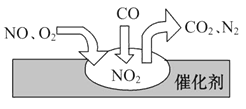
��Ŀ����
����Ŀ�������������ʵ�ת����ϵͼ���ش��й����⣺

��1�����������У�_________����ṹ��ʽ����ͬ���IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־�����������Ե�ζƷ�к���3%~5%��_________��������ζ����״Һ����_____________��
��2����ϩ�Ĺ�ҵ�Ʒ���_________��
a��ʯ���ѻ� b��ú���� c��ʯ���ѽ� d��ʯ�ͷ���
��3����ȩ�еĹ�����������______������ȩ��һ��̼ԭ�ӵ�ͬϵ��Ľṹ��ʽ��______��
��4����ҵ������ϩˮ�������Ҵ�����ϩ�ڼ��ȡ���ѹ�ʹ������ڵ������£���ˮ��Ӧ�����Ҵ����÷�Ӧ�Ļ�ѧ����ʽ��_______________________��
��5���������������Ļ�ѧ����ʽ��__________________________��
���𰸡�CH2=CH2 CH3COOH CH3COOCH2CH3 c ȩ�� HCHO CH2=CH2+H2O![]() CH3CH2OH CH3COOH+CH3CH2OH
CH3CH2OH CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
��������
��1����ϩ��CH2=CH2���IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־�����������Ե�ζƷ��ʳ�ף����к���3%~5%�Ĵ��ᣨCH3COOH����һ���������ʾ�����ζ��������ζ����״Һ��������������CH3COOCH2CH3����
����CH2=CH2 ��CH3COOH��CH3COOCH2CH3
��2��a��ʯ���ѻ�����һ���������£�����Է��������ϴе�ϸߵ�������Ϊ��Է���������С���е�ϵ͵����Ĺ��̣��õ���С�������ʵ���Է���������Ȼ�ϴ�Ҫ�ٽ�һ���ѽ���ܻ����ϩ����a����
b��ú����ָú�ڸ������������¼��ȡ��ֽ⣬���ɽ�̿����뽹����ú���͡��ֱ���ú���Ȳ���Ĺ��̣�������Ϊ��ȡ��ϩ�ķ�������b����
c��ʯ���ѽ���һ�ָ���ȵ�ʯ���ѻ���ʯ���ѽ����ɵ��ѽ����dzɷָ��ӵĻ�����壬��Ҫ��Ʒ��ϩ����c��ȷ��
d��ʯ�ͷ����ǽ�ʯ���м��ֲ�ͬ�е�Ļ��������һ�ַ��������������仯���õ��������Ȼ�ǻ���������Ϊ��ȡ��ϩ�ķ�������d ����
��ѡc��
��3����ȩ�еĹ�����������ȩ��������ȩ��һ��̼ԭ�ӵ�ͬϵ���Ǽ�ȩ���ṹ��ʽ��HCHO��
��Ϊ��ȩ����HCHO��
��4����ҵ������ϩˮ�������Ҵ�����ϩ�ڼ��ȡ���ѹ�ʹ������ڵ������£���ˮ��Ӧ�����Ҵ����÷�Ӧ�Ļ�ѧ����ʽ��CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��
����CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��
��5���Ҵ���������Ũ������ȵ�����������������������ѧ����ʽ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
����CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�����Ŀ��Zn��һ��Ӧ�ù㷺�Ľ���������п��(��Ҫ�ɷ�ΪZnS��������SiO2������FeS��CdS��PbS���黯�������ʵ�)Ϊԭ���Ʊ�����Zn��ZnSO4��7H2O��������ͼ��ʾ��

����ؽ�������[c(Mn��)��0.1 mol��L��1]�γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Zn2+ | Cd2+ |
��ʼ������pH | 1.5 | 6.3 | 6.2 | 7.4 |
������ȫ��pH | 2.8 | 8.3 | 8.2 | 9.4 |
��FeAsO4������ˮ��ZnSO4��7H2O������ˮ�������ھƾ���
�ش��������⣺
(1)����1����Ҫ�ɷֳ�SiO2���______�����պ����������Ի�����ɵij���Σ��Ϊ______��
(2)�������ӹ����м���ZnO��������___________��
(3)�Ƶõ�ZnSO4��7H2O��ϴ�ӣ�ϴ�Ӿ���ʱӦѡ�õ��Լ�Ϊ____________��
(4)��Һ�е�Cd2������п�۳�ȥ����ԭ���ӹ����з�Ӧ�����ӷ���ʽΪ___________������ʡȥ����ԭ���������裬ֱ���������������������г�ȥCd2����������________��
(5)�������õ�Cd�������������Ӽ��Զ��ε�أ���ع���ʱ������NiO(OH)ת��ΪNi(OH)2������ʱ��ص�������ӦʽΪ_____________������п��ĵ��Һ�ɷ���______�������ʹ�á�
(6)���Һ����Ԫ����AsO33�����ڣ�����������ʱ��������KMnO4��Һ��KMnO4����AsO33��������Ӧ����FeAsO4��д���÷�Ӧ�����ӷ���ʽΪ___________��