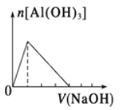
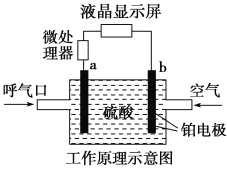
题目内容
【题目】已知下列热化学方程式:则CH4的燃烧热为( )
![]() CH4(g)+O2(g)=
CH4(g)+O2(g)= ![]() CO2(g)+H2O(l) ΔH=-445.15 kJ·mol-1
CO2(g)+H2O(l) ΔH=-445.15 kJ·mol-1
CH4(g)+![]() O2(g)=CO(g)+2H2O(l) ΔH=-607.3 kJ·mol-1
O2(g)=CO(g)+2H2O(l) ΔH=-607.3 kJ·mol-1
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ΔH=-890.3 kJ·mol-1
CH4(g)+2O2(g)=CO2(g)+2H2O(g) ΔH=-802.3 kJ·mol-1
A.445.15 kJ·mol-1B.890.3 kJ·mol-1
C.607.3 kJ·mol-1D.802.3 kJ·mol-1
【答案】B
【解析】
在101 kPa时,1 mol物完全燃烧生成稳定的化合物时所放出的热量该物质的燃烧热。则表示甲烷燃烧热的热化学方程式为CH4(g)+2O2(g)=CO2(g)+2H2O(l) ΔH=-890.3 kJ·mol-1,则CH4的燃烧热为890.3 kJ·mol-1。
答案选B。

【题目】一种“氢氧化锶-氯化镁法”制备“牙膏用氯化锶(SrCl2·6H2O)”的工艺流程如图:
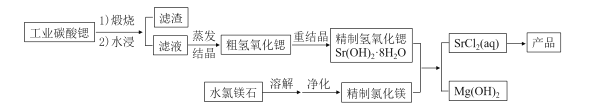
(1)锶与钙同主族。金属锶应保存在__中(填“水”、“乙醇”或“煤油”)。
(2)天青石(主要成分SrSO4)经过多步反应后可制得工业碳酸锶。其中第一步是与过量焦炭隔绝空气微波加热还原为硫化锶,该过程的化学方程式为___。
(3)工业碳酸锶中含有CaCO3、MgCO3、BaCO3等杂质。“滤渣”的主要成分是___。
(4)“重结晶”时蒸馏水用量[以质量比m(H2O):m(SrO)表示]对Sr(OH)2·8H2O纯度及产率的影响如下表。最合适的质量比为___,当质量比大于该比值时,Sr(OH)2·8H2O产率减小,其原因是___。
质量mH2O:mSrO | 4:1 | 5:1 | 6:1 | 7:1 | 8:1 | 9:1 | 10:1 |
Sr(OH)2·8H2O纯度% | 98.64 | 98.68 | 98.65 | 98.64 | 98.63 | 98.63 | 98.65 |
Sr(OH)2·8H2O产率% | 17.91 | 53.36 | 63.50 | 72.66 | 92.17 | 89.65 | 88.93 |
(5)水氯镁石是盐湖提钾后的副产品,其中SO42-含量约为1%,“净化”过程中常使用SrCl2除杂,写出该过程的离子方程式___
(6)若需进一步获得无水氯化锶,必须对SrCl2·6H2O(M=267g·mol-1)进行脱水。脱水过程采用烘干法在170℃下预脱水,失重达33.7%,此时获得的产物化学式为___。